��Ŀ����
����Ŀ��ij�л�������A�����ϣ��������к�̼Ϊ70.6%������Ϊ5.9%���ຬ�������������з����ⶨ���л����������Է��������ͷ��ӽṹ��
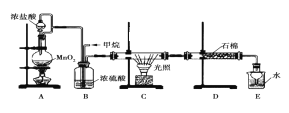
����һ:������������֪A������ͼ��ͼ�£�
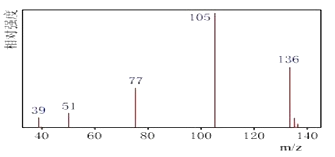
������: �˴Ź����Dz��A�ĺ˴Ź���������ͼ��
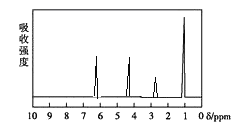
������: ���ú�������Dz��A���ӵĺ�����ף�����ͼ��
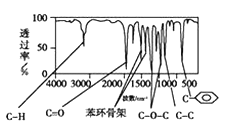
��֪��A�����б�����ֻ��һ��ȡ�������Իش��������⡣
��1��A���ӵ���Է�������_______________������____________�ֻ�ѧ������ͬ����ԭ�ӡ�
��2��A�ķ���ʽΪ_______________________��
��3�����л��ﺬ�еĹ�������______________________��
��4��A�ķ�����ֻ��һ������ֱ��������________________(�����)��
a��A����Է������� b��A�ķ���ʽ
c��A�ĺ˴Ź�������ͼ d��A���ӵĺ������ͼ
��5��A�Ľṹ��ʽΪ_______________________________��
���𰸡�136 4 C8H8O2 ���� bc ![]()
��������
��1����A������ͼ��֪��A����Է�����Ϊ136����A�ĺ˴Ź�������ͼ��֪��A��4���壬��A����4�ֻ�ѧ������ͬ����ԭ�ӣ��ʴ�Ϊ��136��4��
��2��N(C)��N(H)��N(O)=70.6%/12��5.9%/1��(1-70.6%-5.9%)/16=4��4��1����A��ʵ��ʽΪC4H4O����A�ķ���ʽΪ(C4H4O)n����Mr(A)=136���ɵ�n=2����A�ķ���ʽΪ��C8H8O2��
�ʴ�Ϊ��A�ķ���ʽΪ��C8H8O2��
��3����A���ӵĺ������֪��A���б�����ռ6��Cԭ����������C=O��C-O-C��C-C��C-H������C=O��C-O-C�����Ϊ![]() �����Ը�����Ϊ������
�����Ը�����Ϊ������
�ʴ�Ϊ��������
��4�����������2���Ľ������ٽ��A���ӵĺ˴Ź������ף���4����ԭ�ӣ��������Ϊ1��1��1��3��������-CH3����ԭ����Ϊ3������ֻ����һ��-CH3������A�ķ�����ֻ��һ����������Ϊbc���ʴ�Ϊ��bc��
(5)��A���ӵĺ������ͼ��֪��A�����к���һ������������һ��������ֻ����һ�������������Ϸ�����֪��A�Ľṹ��ʽΪ��![]() ��
��
�ʴ�Ϊ��![]() ��
��

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�����Ŀ��X��Y��Z��WΪ������Ԫ�أ���Wԭ�ӵ����������������ڲ���ӵ�![]() ����ͼ��ʾ������˵������ȷ����
����ͼ��ʾ������˵������ȷ����
X | Y | |
Z | W |
A.XԪ�ص��⻯����Ӽ�����γ����
B.YԪ�ص�����ͬ�������峣���¶�������
C.�����Ӱ뾶�Ӵ�С��˳��ΪX>Y>Z>W
D.����������Ӧ��ˮ��������ԣ�W>Z
����Ŀ����þ����һ�ֹ�ҵ���ϣ���Ҫ�ɷ���MgO��ռ40%������������������CaO��MnO��Fe2O3��FeO��Al2O3��SiO2�����ʣ��Դ�Ϊԭ����ȡ������þ��������ӡȾ����ֽ��ҽҩ�ȹ�ҵ������þ������ȡMgSO47H2O�Ĺ����������£�
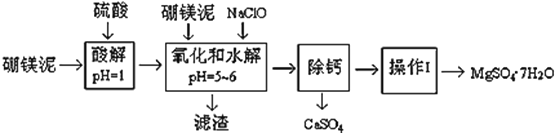
��1��ʵ������Ҫ1 mol/L������800 mL������ 98% ��Ũ���ᣨ��= 1.84 g/mL�������ƣ���ȡŨ������Ҫʹ����Ͳ�Ĺ��Ϊ__________����дѡ����ĸ��
A��10 mL B��20 mL C��50 mL D��100 mL
��2�������NaClO����Mn2+ ��Ӧ��Mn2+ + ClO + H2O = MnO2��+ 2H+ + Cl ���ڸò����л���һ������Ҳ�ᱻNaClO�������÷�Ӧ�����ӷ���ʽΪ___________________��
��3����������Ҫ�ɷֳ�����Fe(OH)3��Al(OH)3�⣬������__________��___________��
��4���ڡ����ơ�ǰ���������Һ��Fe3+ �Ƿ������������鷽��___________________����д������������ͽ��ۣ�
��5����֪MgSO4��CaSO4 ���ܽ�ȣ���λΪ g/100 g ˮ�����±���
�¶ȣ��棩 | 40 | 50 | 60 | 70 |
MgSO4 | 30.9 | 33.4 | 35.6 | 36.9 |
CaSO4 | 0.210 | 0.207 | 0.201 | 0.193 |
�����ơ��ǽ�MgSO4��CaSO4�����Һ�е�CaSO4��ȥ�������ϱ����ݣ���Ҫ˵����������______�����������ǽ���Һ��������Ũ������ȴ�ᾧ��______����õ���MgSO47H2O
��6��ʵ�����ṩ����þ�100 g���õ� MgSO47H2OΪ172.2 g ����MgSO47H2O �IJ���Ϊ___��