��Ŀ����
����Ŀ���״���һ����Ҫ�Ŀ�������Դ��
����֪��2CH4(g)+O2(g)=2CO(g)+4H2(g) ��H=��70.8 kJ��mol��1 CO(g)+2H2(g)=CH3OH(g) ��H=��90.5 kJ��mol��1
д����CH4��O2��ȡCH3OH(g)���Ȼ�ѧ����ʽ_____________________��
���ڷ�ӦCO(g)+2H2(g)=CH3OH(g)���ش��������⣺
(1)ͼ1��CO(g)��CH3OH(g)���ʵ���Ũ����ʱ��(t)�ı仯���ߣ��ӷ�Ӧ��ʼ���ﵽƽ��ʱ����H2��ʾ�ķ�Ӧ������(H2)=________��CO��ת����Ϊ_________��
(2) ���ݻ�Ϊ2L�ĸ��������г���5mol CO��10mol H2��������Ӧ���ﵽƽ�⣬CO��ƽ��ת�������¶�(T)�ı仯������ͼ2��ʾ��
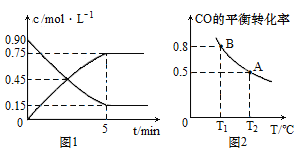
�ټ���B��ƽ�ⳣ��KB��______���ﵽƽ��ʱ�����ٳ���2mol CO��4mol H2��2mol CH3OH����Ӧ��__________����(��������Ӧ�����������淴Ӧ������)��
�ڱȽ�KA��KB�Ĵ�С________��
�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬����____������ĸ��ţ���
A��H2������������ٸı�
B��H2���������ʵ���CH3OH���������ʵ�2��
C��H2��ת���ʺ�CO��ת�������
D����������ƽ����Է����������ٸı�
���𰸡�2CH4(g)+O2(g)= CH3OH(g) ��H=��251.8 kJ ��mol��1 0.3mol��L��1 ��min��1 83.3�� 4 ����Ӧ���� KA��KB AD
��������
���ø�˹�����������ʱ䣬д���Ȼ�ѧ����ʽ��
�� (1)����ͼ1��ʾ��Ũ�������Ϊ�������Ũ�ȱ仯���ߣ���ΪCH3OH��Ũ�ȱ仯���ߣ���Ũ�Ƚ��͵�ΪCO��Ũ�ȱ仯���ߣ��ҵ�Ũ�ȵı仯���������(CH3OH)�����ݷ�Ӧ����֮�ȵ��ڻ�ѧ������֮�ȣ��õ���(H2)=2��(CH3OH)������ͼ1��ʾ���ҵ�CO��ת��Ũ�Ⱥ���ʼŨ�ȣ���ת��Ũ������ʼŨ�ȵı�ֵ�ɵõ�ת���ʣ�
(2) ��������ʽ���ƽ�ⳣ�������ٳ���2mol CO��4mol H2��2mol CH3OH������Ũ���̣���K�Ƚ��жϷ�Ӧ���еķ���
�ڴ�T1��T2�������¶ȣ�CO��ת���ʼ�С������Ӧ�����ƶ���ƽ�ⳣ����С����KA<KB��
���ж��Ƿ���ƽ��״̬���ɸ������淴Ӧ�����Ƿ���ȣ������ʵ�Ũ���Ƿ��ֱ���оݣ�Ҳ���Ը��ݱ���������������
��.��2CH4(g)+O2(g)=2CO(g)+4H2(g)��H=��70.8 kJ/mol
��CO(g)+2H2(g)=CH3OH(g)��H=��90.5 kJ/mol
���ݸ�˹���ɣ��ɢ�+����2�ã�2CH4(g)+O2(g)= CH3OH(g) ��H=��251.8 kJ ��mol��1��
�ʴ�Ϊ��2CH4(g)+O2(g)= CH3OH(g) ��H=��251.8 kJ ��mol��1��
��.(1)����ͼ1��ʾ��Ũ�������Ϊ�������Ũ�ȱ仯���ߣ���ΪCH3OH��Ũ�ȱ仯���ߣ���Ũ�Ƚ��͵�ΪCO��Ũ�ȱ仯���ߣ���Ӧ5���Ӻ�ﵽƽ��״̬������(CH3OH)=![]() =0.15mol��L��1��min��1������(H2)=2��(CH3OH)= 0.15mol��L��1��min��1��2=0.3mol��L��1��min��1������ͼ1��ʾ��CO��ʼŨ��Ϊ0.9 mol��L��1��ƽ��ʱ��Ũ��Ϊ0.15mol��L��1����CO��Ũ�ȱ仯��Ϊ0.9 mol��L��1-0.15mol��L��1=0.75 mol��L��1�����CO��ת����Ϊ
=0.15mol��L��1��min��1������(H2)=2��(CH3OH)= 0.15mol��L��1��min��1��2=0.3mol��L��1��min��1������ͼ1��ʾ��CO��ʼŨ��Ϊ0.9 mol��L��1��ƽ��ʱ��Ũ��Ϊ0.15mol��L��1����CO��Ũ�ȱ仯��Ϊ0.9 mol��L��1-0.15mol��L��1=0.75 mol��L��1�����CO��ת����Ϊ![]() =83.3����
=83.3����
����0.3mol��L��1 ��min��1��83.3����
(2) ����ͼ2��ʾ��T1�¶��£�B��ﵽƽ��ʱCO��ת����Ϊ0.8����CO������0.8��5mol=4mol�����Ϸ�Ӧ������ʽ��
CO(g)+2H2(g)![]() CH3OH(g)
CH3OH(g)
��ʼ(mol) 5 10 0
�仯(mol) 4 8 4
ƽ��(mol) 1 2 4
��B��ƽ�ⳣ��KB��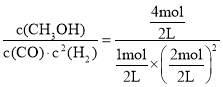 =4���ﵽƽ��ʱ�����ٳ���2mol CO��4mol H2��2mol CH3OH��Ũ����Qc=
=4���ﵽƽ��ʱ�����ٳ���2mol CO��4mol H2��2mol CH3OH��Ũ����Qc=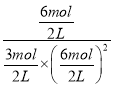 =
=![]() <4����Ӧ������Ӧ������У�
<4����Ӧ������Ӧ������У�
��Ϊ��4������Ӧ����
�ڴ�T1��T2�������¶ȣ�CO��ת���ʼ�С������Ӧ�����ƶ���ƽ�ⳣ����С����KA<KB��
����KA<KB��
��A��H2������������ٸı��ǻ�ѧƽ����������ﵽ��ƽ�⣻
B��H2���������ʵ���CH3OH���������ʵ�2��������ָ����Ӧ������˵�����淴Ӧ������ȣ���һ��ƽ�⣻
C��CO��H2��ʼ���ʵ���֮��Ϊ1:2��ת�����ʵ���֮��Ϊ1:2����ϵ��H2��ת���ʺ�CO��ת���ʺ���ȣ���ϵ��H2��ת���ʺ�CO��ת������Ȳ���˵�����淴Ӧ������ȣ�
D����������ƽ����Է��������������������ʵ����ı�ֵ�����ʵ����仯���������䣬���Ե���ϵ�������ƽ����Է����������ٸı䣬֤���ﵽ��ƽ�⣻
��ѡAD��

����Ŀ��һ���¶��£������������Ϊ0.5 L�ĺ����ܱ������з�����Ӧ��CO(g)+Cl2(g) ![]() COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
COCl2(g)�������������з�Ӧ��5 minʱ�ﵽƽ��״̬��
������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | ||
CO | Cl2 | COCl2 | COCl2 | ||
�� | 500 | 1.0 | 1.0 | 0 | 0.8 |
�� | 500 | 1.0 | a | 0 | 0.5 |
�� | 600 | 0.5 | 0.5 | 0.5 | 0.7 |
����˵������ȷ����
A.�÷�Ӧ����ӦΪ���ȷ�Ӧ
B.��������ǰ5 min��ƽ����Ӧ������(CO)=0.16 mol��L-1��min-1
C.��������a��0.55 mol
D.��������Ϊ��ѹ���ﵽƽ��ʱCOת����С��80%
����Ŀ�����������(Na2S2O3)��һ�ֽⶾҩ�����ڷ�����顢����Ǧ����������ж����ٴ�����������ݡ���Ƥ�������Ȳ�֢.��������������Ի���Ի������ȶ�����������Һ�зֽ����S��SO2
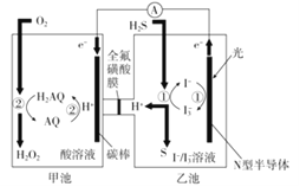
ʵ��I��Na2S2O3���Ʊ�����ҵ�Ͽ��÷�Ӧ��2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2�Ƶã�ʵ����ģ��ù�ҵ���̵�װ����ͼ��ʾ��

(1)����a��������_______������b��������_______��b��������������Ϊ70%80%��H2SO4��Һ��Na2SO3���巴Ӧ�Ʊ�SO2��Ӧ�Ļ�ѧ����ʽΪ_______��c���Լ�Ϊ_______
(2)ʵ����Ҫ����SO2���������ʣ����Բ�ȡ�Ĵ�ʩ��_______ (д��һ��)
(3)Ϊ�˱�֤��������ƵIJ�����ʵ����ͨ���SO2���ܹ�����ԭ����_______
ʵ���̽��Na2S2O3����������ӵ�������ԭ��Ӧ��
���ϣ�Fe3++3S2O32-Fe(S2O3)33-(�Ϻ�ɫ)
װ�� | �Լ�X | ʵ������ |
| Fe2(SO4)3��Һ | ��Ϻ���Һ�ȱ���Ϻ�ɫ��30s����Ϊ��ɫ |
(4)��������ʵ���������ж�����Fe3+��S2O32-��ԭΪFe2+��ͨ��_______(��������Լ�������)����һ��֤ʵ������Fe2+���ӻ�ѧ��Ӧ���ʺ�ƽ��ĽǶȽ���ʵ��������_______
ʵ��궨Na2S2O3��Һ��Ũ��
(5)��ȡһ�������IJ�Ʒ���Ƴ������������Һ�����ü�ӵ������궨����Һ��Ũ�ȣ��÷�����ƽȷ��ȡ������K2Cr2O7(Ħ������Ϊ294gmol-1)0.5880g��ƽ���ֳ�3�ݣ��ֱ����3����ƿ�У���ˮ�����Һ�������������KI���ữ���������з�Ӧ��6I-+Cr2O72-+14H+ = 3I2+2Cr3++7H2O���ټ��뼸�ε�����Һ������������Na2S2O3��Һ�ζ���������ӦI2+2S2O32- = 2I- + S4O62-���������� Na2S2O3��Һ��ƽ�����Ϊ25.00 mL�������궨�������������Һ��Ũ��Ϊ_______molL-1
����Ŀ��ij���������ʵ��̽��SO2�����ʡ�
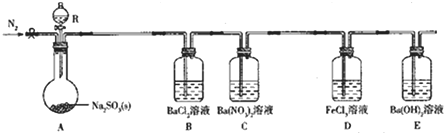
ʵ������B���������ɣ�C���а�ɫ������D����Һ��ɫ��dz��E�в�����ɫ������
��1������R��������___��
��2��ʵ�����ڳ�������80%���������������Ʒ�ĩ��Ӧ�Ʊ�SO2��д��A�з�Ӧ�Ļ�ѧ����ʽ��___��
��3������ʵ�鲽�����£�����װ�á���������ԡ�װҩƷ����װ����ͨ��һ��ʱ��N2��Ȼ������A�з�Ӧ����ͨ��һ��ʱ���N2����Ŀ����___��
��4��̽��װ��C��ͨ��SO2���������Ĺ�ϵ��
������������²��룺
����1��ͨ�������SO2��������Ӧ�����ӷ���ʽΪBa2++2NO3-+3SO2+2H2O=BaSO4��+2SO42-+2NO+4H+��
����2��ͨ��������SO2��������Ӧ�����ӷ���ʽΪ___��
��������������ʵ��֤������1�Ͳ���2�ĸ��������������ʵ�飺
�ṩ�Լ�����ˮ��ͭ�ۡ�Ba(NO3)2��Һ��BaCl2��Һ��Na2CO3��Һ
ʵ�鲽�� | ʵ�������� |
ȡ����C�з�Ӧ����Һ���Թ��У�___ | ___ |
��5�����пɼ���װ��D��Ӧ����Һ���Ƿ���Fe2+������Լ���___(����ĸ)��
a.KSCN��Һ b.NaOH��Һ c.K3[Fe(CN)6]��Һ d.KSCN��Һ��˫��ˮ
��6�������£�ʵ����Ϻ��롢�ᴿװ��E�а�ɫ����M��ȡ����M���Թ��У�����������(Mʣ��)��������Һ��pH___7(����>����<������=��)��