��Ŀ����
18-�� ���������������ڵ������͡�����ܵ�ʳ���㾫����ϳɷ�Ӧ�Ļ�ѧ����ʽ���£�
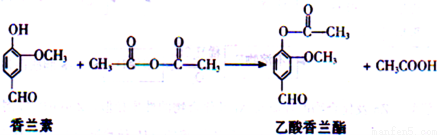

����������ȷ���ǣ�_____��
A���÷�Ӧ����ȡ����Ӧ
B�������������ķ���ʽΪC10H8O4
C��FeCl3��Һ����������������������������
D������������������NaOH��Һ��ˮ��õ������������
18-���� A��CH2=CH2���� D(HOOCCH=CHCH=CHCOOH)�ϳɸ߷���P����ϳ�·�����£�
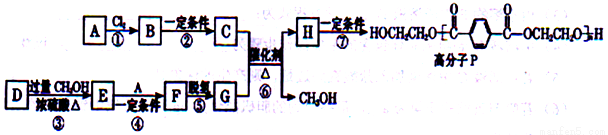
��֪����
�����봼�ɷ���������������Ӧ��

��1��B������Ϊ_________________��D�й����ŵ�����Ϊ_________________________��
��2��C�ķ���ʽ��C2H6O2����Ӧ�ڵ��Լ��ͷ�Ӧ������____________________________��
��3��F�Ľṹ��ʽ��__________________________��
��4����Ӧ�Ļ�ѧ����ʽ��____________________________________��
��5��G��һ��ͬ���칹��G'Ϊ���������˴Ź���������3�ַ���1mol���л�������������ˮ���������2 mol NaOH��Ӧ��G'�Ľṹ��ʽΪ___________________��
��6���ԶԱ����״����״�Ϊ��ʼԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�G��д���ϳ�·�ߣ��ýṹ��ʽ��ʾ�л���ü�ͷ��ʾת����ϵ����ͷ��ע���Լ��ͷ�Ӧ��������___________________
�����ʾΪԪ�����ڱ���һ���֣�����Ԫ�آ١����ڱ��е�λ�ã���ش��������⣺
�� ���� | IA | 0 | ||||||
1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� | ||||
��1���ۡ��ܡ��ߵ�ԭ�Ӱ뾶�ɴ�С��˳����_________����Ԫ�ط��ű�ʾ����
��2��������ʵ��˵����Ԫ�صķǽ����ԱȢ�Ԫ�صķǽ�����ǿ����__________��
a.�ڵĵ������Ԫ�صļ��⻯����Һ��Ӧ����Һ�����
b.��������ԭ��Ӧ�У�1mol�ڵ��ʱ�1mol���ʵõ��Ӷ�
c.�ں͢���Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸߡ�
��3���١�������Ԫ�ذ�ԭ�Ӹ���֮��Ϊ1��1��ɵij���Һ̬�������������Һ���ܽ�Fe2+ ������д���÷�Ӧ�����ӷ���ʽ ___________________��
��4�� ��֪���ڱ��д��ڶԽ����ƹ������루Be��������ѧ�������ƣ�����������������Ҳ�����ԣ�д���������������ܵ�����������ˮ���ﷴӦ�Ļ�ѧ����ʽ _______________________��
��5����֪W+X=Y+Z����Ӧ��Ҫ���ȣ�����W��X��Y��Z�ֱ����ɢ٢ڢ�����Ԫ���γɵ�����10�������ӣ�W��XΪ���ӣ�Y��ZΪ���ӣ���д���û�ѧ����ʽ_________________��
��6���ɱ���Ԫ���γɵ����ʿɷ�����ͼ�еķ�Ӧ������B��C��G�ǵ��ʣ�BΪ����ɫ���壬 D��Һ�Լ��ԡ�
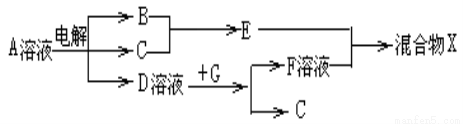
��д��D��Һ��G��Ӧ�����ӷ���ʽ______________________��
��д������A��Һ�����ʵ������ӵķ���____________________��
�۳����£������1L0.1mol/L��A��Һ��һ��ʱ�������ҺpHΪ12��������Һ����仯������õ�������ת�Ƶ��ӵ����ʵ���Ϊ��________________��
��������H2��ԭCuCl�Ʊ�����ͭ����Ӧԭ�����£�
2Cu(s)+Cl2 (g) 2CuCl(s) ��H1=-36 kJ��mol-1 ��
2CuCl(s) ��H1=-36 kJ��mol-1 ��
H2(g)+2CuCl( s)=2Cu(s)+2HCl(g) ��H2 ��
�й����ʵļ����������±���
���� | H2 | Cl2 | HCl |
����(kJ��mol-1) | 436 | 243 | 432 |
(1)���H2=_______ kJ��mol-1��
(2)���ⶨ��Ӧ���Ʊ�����ͭ�ķ�Ӧ���ƴ�ԭ����_______________��
(3)��ij�¶��£���Ӧ�ٴﵽƽ��״̬����tlʱ������ѹǿ��ԭ����2����Cu�����㹻������ͼ�л���Cl2Ũ�ȵı仯�����ߡ�_______________
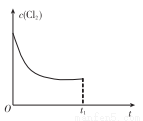
(4)��ɫ������ˮ��CuCl�����ɵ�ⷨ�Ƶã���ͼ��ʾ��
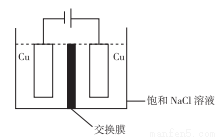
��װ�����õĽ���ĤΪ_____________��
A.�����ӽ���Ĥ B�������ӽ���Ĥ C�����ӽ���Ĥ D�����������ӽ���Ĥ
�������ĵ缫��ӦʽΪ_________________��
(5)��֪CuCl���ܽ���ϡ���ᣬд���÷�Ӧ�Ļ�ѧ����ʽ��_________________��
(6)������ѧ֪ʶд����ȡCuCl��һ�ַ������û�ѧ����ʽ��ʾ��_________________��



 Mg2+(aq) + 2OH��(aq)���ù��������NH4Cl��Һ
Mg2+(aq) + 2OH��(aq)���ù��������NH4Cl��Һ