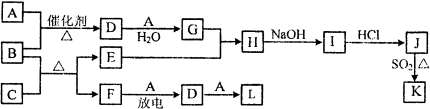
��Ŀ����
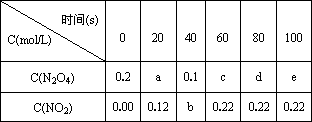
��80��ʱ����0.20mol�������������������1L�ѳ�յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
|
| 0 | 20 | 40 | 60 | 80 | 100 | ||
| C(N2O4)��mol/L�� | 0.20 | C1 | 0.10 | C3 | A | B | ||
| C(NO2) ��mol/L�� | 0.00 | 0.12 | C2 | 0.22 | 0.22 | 0.22 |
���ݱ������ݺͱ������ṩ�����ݣ�![]() ��
��![]() ��
��![]() ��A��B��ʾ��Ӧ��Ũ�ȣ��ش����и�С�⣺
��A��B��ʾ��Ӧ��Ũ�ȣ��ش����и�С�⣺
(1���÷�Ӧ�Ļ�ѧ����ʽΪ__________���ﵽƽ��ʱ������������ת����Ϊ_____%������![]() ���<������=������>�� ����
���<������=������>�� ����
(2��20sʱ��������������Ũ��![]() ����0s�D20s��������������ƽ����Ӧ����Ϊ___________��
����0s�D20s��������������ƽ����Ӧ����Ϊ___________��
(3��������ͬ���������������������Ƕ����������壬Ҫ�ﵽ����ͬ����ƽ��״̬��������������ʼŨ����![]() ��
��
(1)N2O4 ![]() 2NO2 55 > =
2NO2 55 > =
(2)0.14 0.003 mol/(L?s)
(3)0.40

| |||||||||||||||||||||||||||
 ��80��ʱ����0.40mol��N204�������2L�Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4?2NO2����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
��80��ʱ����0.40mol��N204�������2L�Ѿ���յĹ̶��ݻ����ܱ������У��������·�Ӧ��N2O4?2NO2����һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
 2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�
2NO2 ������ɫ������80��ʱ����0.80mol��N2O4�������4L�Ѿ���յĹ̶��ݻ����ܱ������У���һ��ʱ��Ը������ڵ����ʽ��з������õ��������ݣ�