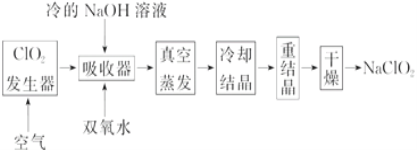
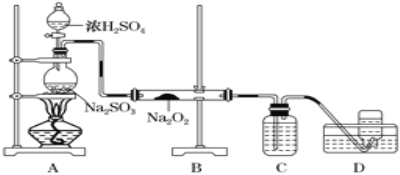
��Ŀ����
����Ŀ��ij����ˮ�к�5.00��10-3mol��L-1��![]() ���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���
���䶾�Խϴ�ij�о���ѧϰС��Ϊ�˱��Ϊ��������ˮ�����õ����Բ���![]() ��
��![]() �Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�
�Ļ��ϼ�����Ϊ+3��+2�������������ʵ�����̣�

��1����������Ӧ�����ӷ���ʽ��_________________________________________________��
��2������������pH��ֽ�ⶨ��ҺpH�IJ����ǣ�
______________________________________________________________________________��
��3�����������˵õ�����������Ҫ�ɷֳ�Cr��OH��3�⣬����______________________��
��4����ʹ1L�÷�ˮ�е�![]() ��ȫת��Ϊ
��ȫת��Ϊ![]() ����������Ҫ����__________g FeSO4��7H2O��
����������Ҫ����__________g FeSO4��7H2O��
���𰸡�Cr2O72-+ 6Fe2��+ 14H��![]() 2Cr3+ + 6Fe3��+ 7H2O ��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ������ Fe(OH)3��Fe(OH)2 13.9
2Cr3+ + 6Fe3��+ 7H2O ��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ������ Fe(OH)3��Fe(OH)2 13.9
��������
(1)Cr2O72-�н�ǿ�����ԣ�FeSO47H2O��Fe2+��һ���Ļ�ԭ�ԣ������Խ����з���������ԭ��Ӧ����ʵ�����̿�֪���ڢٲ���Ӧ��Cr2O72-�����������½�Fe2+����ΪFe3+����������ԭΪCr3+�������غ�Ԫ���غ㼰����������֪����Ӧ��ˮ���ɣ���Ӧ���ӷ���ʽΪCr2O72-+14H++6Fe2+=2Cr3++6Fe3++7H2O���ʴ�Ϊ��Cr2O72-+14H++6Fe2+=2Cr3++6Fe3++7H2O��
(2)��һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��ʴ�Ϊ����һС��pH��ֽ���ڱ������ϣ��ò�����պȡ��������Һ������pH��ֽ�ϣ��������ɫ�����գ�
(3)�������ͼ�ɵã�Fe2+��������NaOHʱ������Cr(OH)3��Fe(OH)3��Fe(OH)2���ֳ�����ʴ�Ϊ��Fe(OH)3��Fe(OH)2��
(4)1 L��ˮ�к�n(Cr2O72-)=5.00��10-3 mol������Crԭ�ӡ�Feԭ���غ㣬�ɵã�Cr2O72-������4Cr0.5Fe1.5FeO4������10FeSO47H2O������������n(FeSO47H2O)=10n(Cr2O72-)=5.00��10-3 mol��10=0.05 mol������m(FeSO47H2O)=0.05 mol��278 g/mol=13.9 g���ʴ�Ϊ��13.9g��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijѧ������֪���ʵ���Ũ�ȵ��������ⶨδ֪���ʵ���Ũ�ȵ�NaOH��Һʱ��ѡ�������ָʾ��������д���пհס�
��1���ñ�������ζ������NaOH��Һʱ����������ʽ�ζ��ܵĻ���������ҡ����ƿ���۾�ע��____��ֱ�������һ���������Һ��______ɫ��Ϊ____ɫ����____Ϊֹ��
��2�����в����п���ʹ����NaOH��Һ��Ũ����ֵƫ�͵���__________��
A.��ʽ�ζ���δ�ñ�������ϴ��ֱ��ע�������
B.�ζ�ǰʢ��NaOH��Һ����ƿ������ˮϴ����û�и���
C.��ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ
D.��ȡ�������ʱ����ʼ���Ӷ������ζ�����ʱ���Ӷ���
��3�����ζ���ʼ�ͽ���ʱ����ʽ�ζ����е�Һ����ͼ��ʾ������ʼ����Ϊ_____mL������������Һ�����Ϊ_____mL��

��4��ijѧ������3��ʵ��ֱ��¼�й����������
�ζ� ���� | ����NaOH��Һ�����/mL | 0.1000mol��L��1��������/mL | ||
�ζ�ǰ���� | �ζ������ | ��Һ���/mL | ||
��һ�� | 25.00 | 0.00 | 25.11 | 25.11 |
�ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
������ | 25.00 | 0.22 | 25.31 | 25.09 |
���ݱ���������ʽ�����NaOH��Һ�����ʵ���Ũ��_____��������λС������
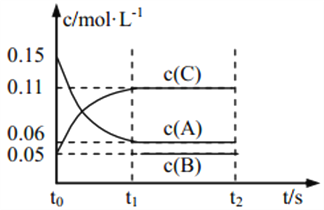
����Ŀ����25��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����������˵���������ǣ��� ��
���� | X | Y | Z |
��ʼŨ��/ | 0.1 | 0.2 | 0 |
ƽ��Ũ��/ | 0.05 | 0.05 | 0.1 |
A.��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50��
B.��Ӧ�ɱ�ʾΪX+3Y![]() 2Z����ƽ�ⳣ��Ϊ1600
2Z����ƽ�ⳣ��Ϊ1600
C.�ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��
D.��ѹʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������