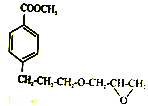
��Ŀ����
����Ŀ��Ϊ�ⶨijNaHCO3��Ʒ�Ĵ��ȣ�����ΪNaCl������������ʵ�飺
��1����ȡ2.000g��Ʒ�����200mL����Һ�����ƴ���Һ���趨����������
��2������ƿ�з���20.00mL����Һ���μ�2�μ��ȣ�ҡ�ȣ���0.100mol/L�ı�����ζ����ߵα�ҡ����ƿ���۾�ע�� �� ֱ���������һ�����ᣬ��Һ��ɫ��ɫ��Ϊɫ�������ڲ������仯��ֹͣ�ζ�����¼������
��3���ظ��ζ�ʵ��1��2�Σ�����ƽ����������22.60mL���ظ�ʵ���Ŀ���� ��
��4���������Һ��NaHCO3��Ũ��Ϊmol/L����Ʒ��NaHCO3����������Ϊ ��
���𰸡�
��1��������ƽ��200mL����ƿ
��2����ƿ����Һ��ɫ�仯���ƣ��ȣ������
��3������ʵ������в��������,ʹ�òⶨ�����ȷ
��4��0.1130��94.92%
���������⣺��1����ȡ2.000g��Ʒ����Ҫ��ȷ�ȸߵķ�����ƽ�����200mL����Һ����Ҫ200ml������ƿ�����ƴ���Һ���趨�������У�������ƽ��200ml����ƿ��
���Դ��ǣ�������ƽ��200mL����ƿ����2������ƿ�з���20.00mL����Һ���μ�2�μ��ȣ�ҡ�ȣ���0.100mol/L�ı�����ζ����ߵα�ҡ����ƿ���۾�ע����ƿ����Һ��ɫ�仯��ֱ���������һ�����ᣬ�ζ��յ�ʱ��Һ��ɫ�ɻ�ɫͻ��Ϊ��ɫ���Ұ�����ڲ���ɫ��ֹͣ�ζ�����¼������
���Դ��ǣ���ƿ����Һ��ɫ�仯���ƣ��ȣ�����ӣ���3���ظ��ζ�ʵ��1��2�Σ�����ƽ����������22.60mL���ظ�ʵ���Ŀ���Ǽ���ʵ������в�������ʹ�òⶨ�����ȷ��
���Դ��ǣ�����ʵ������в�������ʹ�òⶨ�����ȷ����4��ƽ������0.100mol/L����22.60mL��NaHCO3+HCl=NaCl+CO2��+H2O��̼���������ʵ������Ȼ������ʵ�����ͬ��n��NaHCO3��=0.1mol/L��0.02260L=0.00226mol��200mL����Һ�к�̼������0.02260mol��c��NaHCO3��= ![]() =0.1130mol/L��̼��������������=
=0.1130mol/L��̼��������������= ![]() ��100%=94.92%��
��100%=94.92%��
���Դ��ǣ�0.1130��94.92%��