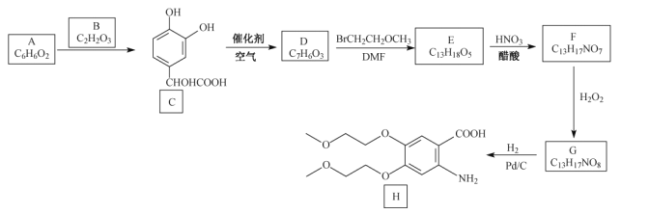
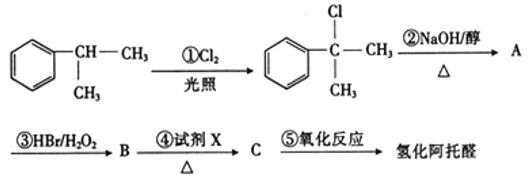
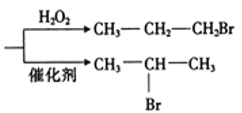
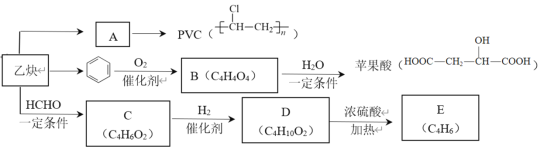
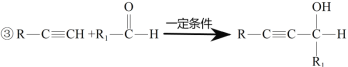
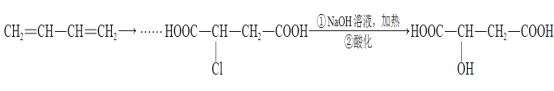��Ŀ����
����Ŀ���⣨Mo�������弰��ֲ��������Ԫ�أ�����оƬ������ҩ���ҽѧ��Ӱ�ȷ���Ҳ����Ҫ���á��û����ұ���������ij��Ӧ��MoS2(s)+2Na2CO3(s)+4H2(g)![]() Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)���÷�Ӧ��������ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)���÷�Ӧ��������ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
(1)Na2S�ĵ���ʽΪ��____________��������Ӧ����̬��Ӧ��������������ڼ��Է��ӵ���____________����д��ѧʽ�����ٳ�һ����ʵ��˵����ķǽ����Ա�̼ǿ���û�ѧ����ʽ��ʾ��____________��
(2)д��������Ӧ��ƽ�ⳣ������ʽK=____________����������Ӧ��____________��Ӧ������ȡ����ȡ�����
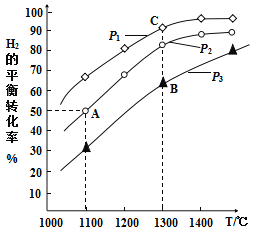
(3)1100�棬2L�����ܱ������У�����0.1molMoS2��0.2molNa2CO3��0.4molH2����Ӧ��20minʱ�ﵽ��ƽ��״̬ǡ�ô�����ͼ�е�A�㡣�˹����У���H2��ʾ��ƽ������Ϊ____________����������һ��ʱ��B�������____________C�㣨����ڡ������ڡ���С�ڡ�����˵�����ɣ�____________��
(4)A��B��C���������ƽ��״̬��ƽ�ⳣ���Ĵ�СΪ��KA____________KB____________KC������ڡ������ڡ���С�ڡ�������˵�����ɣ�____________��
���𰸡���![]() CO��H2O Na2CO3+H2SO4��Na2SO4+H2O+CO2��
CO��H2O Na2CO3+H2SO4��Na2SO4+H2O+CO2�� ![]() ���� 0.005mol/(Lmin) ���� B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�� С�� ���� ƽ�ⳣ��ֻ���¶ȵı仯���仯��B��C���¶���ͬ��KB����KC���÷�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KֵԽ��KAС��KB
���� 0.005mol/(Lmin) ���� B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�� С�� ���� ƽ�ⳣ��ֻ���¶ȵı仯���仯��B��C���¶���ͬ��KB����KC���÷�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KֵԽ��KAС��KB
��������
(1)Na2SΪ���ӻ������Na+��S2-���ɣ��ɴ˿�д�������ʽ��������Ӧ����̬��Ӧ����������й���H2��CO��H2O���֣�������֪��Щ���ڼ��Է��ӡ�˵����ķǽ����Ա�̼ǿ��������ǿ��������ķ�Ӧ��
(2)��ӦMoS2(s)+2Na2CO3(s)+4H2(g)![]() Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)�У�H2��CO��H2OΪ���壬�ɱ�ʾ��Ӧ��ƽ�ⳣ������ʽK����H2��ƽ��ת�������¶ȵı仯���ߣ���ȷ������Ӧ���͡�
Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)�У�H2��CO��H2OΪ���壬�ɱ�ʾ��Ӧ��ƽ�ⳣ������ʽK����H2��ƽ��ת�������¶ȵı仯���ߣ���ȷ������Ӧ���͡�
(3) A�㣬H2��ת����Ϊ50%���ɴ˿����H2�����ʵ����ı仯������H2��ʾ��ƽ�����ʡ���������һ��ʱ������H2��ƽ��ת���ʵĹ�ϵ����ȷ��P1��P3�Ĺ�ϵ���ɴ�ȷ��B���������C��Ĺ�ϵ��
(4)�����ó�A��B��C������¶ȹ�ϵ����������ó�����Ӧ�����ͣ��ɴ˿ɵó�A��B��C�����ƽ�ⳣ����ϵ��
(1)Na2S��Na+��S2-���ɣ�����ʽΪ![]() ��������Ӧ����̬��Ӧ��������ﹲ��H2��CO��H2O���֣�H2���ڷǼ��Է��ӣ����ڼ��Է��ӵ���CO��H2O��������ǿ���������ԭ��˵����ķǽ����Ա�̼ǿ��������H2SO4��Na2CO3��Ӧ����ѧ����ʽΪNa2CO3+H2SO4��Na2SO4+H2O+CO2������Ϊ��
��������Ӧ����̬��Ӧ��������ﹲ��H2��CO��H2O���֣�H2���ڷǼ��Է��ӣ����ڼ��Է��ӵ���CO��H2O��������ǿ���������ԭ��˵����ķǽ����Ա�̼ǿ��������H2SO4��Na2CO3��Ӧ����ѧ����ʽΪNa2CO3+H2SO4��Na2SO4+H2O+CO2������Ϊ��![]() ��CO��H2O��Na2CO3+H2SO4��Na2SO4+H2O+CO2����
��CO��H2O��Na2CO3+H2SO4��Na2SO4+H2O+CO2����
(2)��ӦMoS2(s)+2Na2CO3(s)+4H2(g)![]() Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)�У�H2��CO��H2OΪ���壬ƽ�ⳣ������ʽK=
Mo(s)+2CO(g)+4H2O(g)+2Na2S(s)�У�H2��CO��H2OΪ���壬ƽ�ⳣ������ʽK=![]() ��д��
��д��![]() �������߿��������¶ȵIJ������ߣ�H2��ƽ��ת���ʲ���������ƽ�������ƶ���������������Ӧ�����ȷ�Ӧ����Ϊ��
�������߿��������¶ȵIJ������ߣ�H2��ƽ��ת���ʲ���������ƽ�������ƶ���������������Ӧ�����ȷ�Ӧ����Ϊ��![]() ��
��![]() �����ȣ�
�����ȣ�
(3)1100����A��H2��ƽ��ת����Ϊ50%����μӷ�Ӧ��H2�����ʵ���Ϊ0.2mol���˹����У���H2��ʾ��ƽ������Ϊ =0.005mol/(Lmin)����Ϊ��Ӧ�����������С�����������������������ѹǿС��H2��ƽת���ʴ��ɴ˿ɵ�P1<P2<P3����������һ��ʱ��B������ʴ���C�㣬�����ǣ�B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�㡣��Ϊ��0.005mol/(Lmin)�����ڣ�B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�㣻
=0.005mol/(Lmin)����Ϊ��Ӧ�����������С�����������������������ѹǿС��H2��ƽת���ʴ��ɴ˿ɵ�P1<P2<P3����������һ��ʱ��B������ʴ���C�㣬�����ǣ�B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�㡣��Ϊ��0.005mol/(Lmin)�����ڣ�B��C��ѹǿ��ͬ��ѹǿԽ��H2��ת����ԽС������p3>p1��B�����ʴ���C�㣻
(4)ƽ�ⳣ��ֻ���¶�Ӱ�죬�˷�Ӧ������ӦΪ���ȷ�Ӧ���¶ȸ�ƽ�ⳣ�����¶�A<B=C����ƽ��״̬��ƽ�ⳣ���Ĵ�СΪ��KAС��KB����KC�������ǣ�ƽ�ⳣ��ֻ���¶ȵı仯���仯��B��C���¶���ͬ��KB����KC���÷�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KֵԽ��KAС��KB����Ϊ��С�ڣ����ڣ�ƽ�ⳣ��ֻ���¶ȵı仯���仯��B��C���¶���ͬ��KB����KC���÷�Ӧ�����ȷ�Ӧ���¶�Խ�ߣ�KֵԽ��KAС��KB��

 һ����ʦ�����Ծ�ϵ�д�
һ����ʦ�����Ծ�ϵ�д�