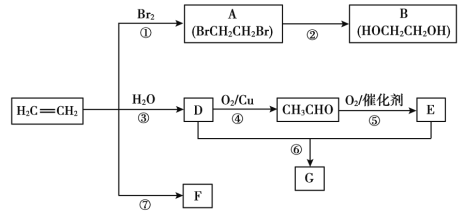
��Ŀ����
����Ŀ������������������������������Ҫ�����á���ش��������⣺
(1)����������H2N2O2��һ�ֶ�Ԫ���ᣬ��ˮ��Һ�л����ֽ⣬��ֽ�ԭ����̼��ֽ�ԭ�����ƣ���д������������ֽ�Ļ�ѧ����ʽ_________________________��
(2)��һϡ�����ϡ����Ļ���ᣬ����H2SO4��HNO3�����ʵ���Ũ�ȷֱ���3 mol��L��1��1 mol��L��1��ȡ100 mL�˻���ᣬ�����м�����������ۣ�����Ӧ�����ɲ�����״���µ���������Ϊ____________ (�跴Ӧ��HNO3����ԭ��NO)��
(3)����ͭ��һ����Ũ���ᷴӦ�õ�����ͭ��Һ��NO2��N2O4��NO�Ļ�����壬��Щ������1.5 molO2��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�����������ᣬ������������ͭ��Һ�м���NaOH��Һ��Cu2+ǡ����ȫ��������������Һ��NaOH�����ʵ���Ϊ____________mol
���𰸡�H2N2O2 =N2O�� + H2O 5.6 L 6
��������
(1)̼������Һ�зֽ����ɶ�����̼��ˮ��̼��Ͷ�����̼��̼Ԫ�ػ��ϼ���ͬ�������������е�Ԫ�ػ��ϼ�Ϊ+1�ۣ���������������ˮ��Һ�зֽ�ԭ����̼��ֽ�ԭ�����ƿ�֪��������������ˮ��Һ�зֽ����ɵ�Ԫ�ػ��ϼ���ͬ��һ����������ˮ��
��2���������֪������������ϡ�����ϡ���ᷴӦ����һ��������������
��3���ɷ�Ӧ���ɵ�NO2��N2O4��NO�Ļ��������1.5 molO2��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�������������֪��ͭʧȥ�������ʵ������������õ����ӵ����ʵ�����
(1)̼������Һ�зֽ����ɶ�����̼��ˮ��̼��Ͷ�����̼��̼Ԫ�ػ��ϼ���ͬ�������������е�Ԫ�ػ��ϼ�Ϊ+1�ۣ���������������ˮ��Һ�зֽ�ԭ����̼��ֽ�ԭ�����ƿ�֪��������������ˮ��Һ�зֽ����ɵ�Ԫ�ػ��ϼ���ͬ��һ����������ˮ����Ӧ�Ļ�ѧ����ʽΪH2N2O2 =N2O�� + H2O���ʴ�Ϊ��H2N2O2 =N2O�� + H2O��
��2��100 mL������������ӵ����ʵ���Ϊ3 mol��L��1��0.1L��2+1mol��L��1��0.1L=0.7mol������������ʵ���Ϊ1 mol��L��1��0.1L=0.1mol������������ϡ���ᷴӦ��������������һ��������ˮ����Ӧ�Ļ�ѧ����ʽΪ3Fe+2NO3-+8H+=3Fe2++2NO��+4H2O���ɷ���ʽ��֪��0.1mol�������ȫ��Ӧ��0.4mol�����ӣ���Ӧ�������ӹ���0.3mol��������������������Ӧ������������״��������һ�����������������Ϊ��0.1mol+![]() ����22.4L/mol=5.6L���ʴ�Ϊ��5.6��
����22.4L/mol=5.6L���ʴ�Ϊ��5.6��
��3���ɷ�Ӧ���ɵ�NO2��N2O4��NO�Ļ��������1.5 molO2��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�������������֪��ͭʧȥ�������ʵ������������õ����ӵ����ʵ�������ͭ���ӵ����ʵ���Ϊ![]() =3mol����ͭԭ�Ӹ����غ��֪��ͭ���ӵ����ʵ���Ϊ3mol����Cu2+��OH����֪��������Һ��NaOH�����ʵ���Ϊ3mol��2=6mol���ʴ�Ϊ��6��
=3mol����ͭԭ�Ӹ����غ��֪��ͭ���ӵ����ʵ���Ϊ3mol����Cu2+��OH����֪��������Һ��NaOH�����ʵ���Ϊ3mol��2=6mol���ʴ�Ϊ��6��

����Ŀ�����Ϊ�١����Ԫ�أ���Ԫ�����ڱ��е�λ�����£�
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
1 | �� | �� | ||||||
2 | �� | �� | �� | �� | ||||
3 | �� | �� | �� | �� |
�Իش��������⣺
��1��(��дԪ�ط���)�� _____��______��_____ ��_____
��2���ٺܺ͢�Ԫ���γɵĻ�����Ļ�ѧʽ________���õ���ʽ��ʾ���γɹ���Ϊ__________��
��3���ߺ͢��Ԫ�ص�����������ˮ����ļ����ǣ� ______��_________ ���ѧʽ������͢��Ԫ�ص�����������ˮ����������ǣ� ______ ��_________ ���ѧʽ����
��4���١��ݡ��ߺ�Ԫ���γɵ�һ�ֻ�����ĵ���ʽ��________���ڸû������мȺ���________�����ֺ���________����