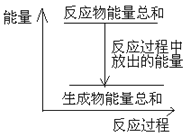
��Ŀ����
����Ŀ��(1)��Ϊ��Ҫ����ԭ�ϣ��й㷺��;���ϳɰ��е������������з�Ӧ��ȡ��
a.CH4(g)+H2O(g)![]() CO(g)+3H2(g) ��H=+216.4KJ/mol
CO(g)+3H2(g) ��H=+216.4KJ/mol
b.CO(g)+H2O(g)![]() CO2(g)+H2(g) ��H=-41.2kJ/mol
CO2(g)+H2(g) ��H=-41.2kJ/mol
��ӦCH4(g)+2H2O(g)![]() CO2(g)+4H2(g) ��H=______
CO2(g)+4H2(g) ��H=______
(2)�Ҵ��Ǿƾ����ϵ���Ҫ�ɷ֣��������Ƿ������ɣ���Ӧ�Ļ�ѧ����ʽΪ��C6H12O6(aq)��2C2H5OH(aq)+2CO2(g)����C6H12O6���ٵ�����Ϊ0.23molL-1h-1ʱ��C2H5OH���ӵ�������__
(3)1mol CH4(l)��ȫȼ������CO2(g)��H2O(1)���ų�����889.6kJ��д����Ӧ���Ȼ�ѧ����ʽ_________ ��
���𰸡���H= +175.2kJ/mol 0.46molL-1h-1 CH4(1)+2O2(g)�TCO2(g)+2H2O(l) ��H= -889.6 kJmol-1
��������
(1)���ݸ�˹���ɷ������
(2)��������֮�ȵ��ڼ�����֮�ȼ��㣻
(3)�����Ȼ�ѧ����ʽ��д�ķ�����д��
(1)a.CH4(g)+H2O(g)CO(g)+3H2(g)��H= +216.4kJ/mol��b.CO(g)+H2O(g)CO2(g)+H2(g)��H= -41.2kJ/mol�����ݸ�˹���ɣ���a+b�ɵã�CH4(g)+2H2O(g)CO2(g)+4H2(g)��H=(+216.4 kJ/mol)+(-41.2 kJ/mol)= +175.2kJ/mol���ʴ�Ϊ��+175.2kJ/mol��
(2)C6H12O6(aq)��2C2H5OH(aq)+2CO2(g)��Ӧ��C6H12O6��С������Ϊ0.23molL-1h-1����������֮�ȵ��ڼ�����֮�ȿ�֪C2H5OH���ӵ�������2��0.23molL-1h-1=0.46molL-1h-1 ���ʴ�Ϊ��0.46 molL-1h-1��
(3)1mol CH4(l)��ȫȼ������CO2(g)��H2O(1)���ų�����889.6kJ�����ȷ�Ӧ����H��0����Ӧ���Ȼ�ѧ����ʽΪCH4(l)+2O2(g)=CO2(g)+2H2O(l) ��H= -889.6kJ/mol���ʴ�Ϊ��CH4(l)+2O2(g)=CO2(g)+2H2O(l)��H= -889.6kJ/mol��

����Ŀ��ijС������H2C2O4��Һ������KMnO4��Һ��Ӧ��̽���������Ի�ѧ��Ӧ���ʵ�Ӱ������ʵ��ʱ���ȷֱ���ȡ������Һ��Ȼ�����Թ���Ѹ������Ͼ��ȣ���ʼ��ʱ��ͨ���ⶨ��ɫ����ʱ�����жϷ�Ӧ�Ŀ�������С����������·�����
��� | H2C2O4��Һ | ����KMnO4��Һ | �¶�/�� | ||
Ũ�ȣ�mol/L�� | ���/mL | Ũ�ȣ�mol/L�� | ���/mL | ||
�� | 0.10 | 2.0 | 0.01 | 4.0 | 25 |
�� | 0.20 | 2.0 | 0.01 | 4.0 | 25 |
�� | 0.20 | 2.0 | 0.01 | 4.0 | 50 |
��1����֪��Ӧ��H2C2O4ת��ΪCO2�ݳ���Ϊ�˹۲쵽��ɫ��ȥ��H2C2O4��KMnO4��ʼ�����ʵ�����Ҫ����Ĺ�ϵΪ��n(H2C2O4)��n(KMnO4) ______________��
��2��̽���¶ȶԻ�ѧ��Ӧ����Ӱ���ʵ������_______ �����ţ���ͬ������̽����Ӧ��Ũ�ȶԻ�ѧ��Ӧ����Ӱ���ʵ������ ________.
��3��ʵ��ٲ��KMnO4��Һ����ɫʱ��Ϊ40s�����Ի��ǰ����Һ�����С�仯�����ʱ����ƽ����Ӧ����v(KMnO4)=_______��