��Ŀ����
����Ŀ���Ķ������������������ϣ�
����һ��
���϶���
���� | �۵�/�� | �е�/�� | �ܶ�/g��cm-3 | �ܽ��� |
�Ҷ����� C2H6O2�� | -11.5 | 198 | 1.11 | ������ˮ���Ҵ� |
��������C3H8O3�� | 17.9 | 290 | 1.26 | �ܸ�ˮ���ƾ�������Ȼ��� |
�ش��������⣨��д��ţ���
A������ | B����ȡ�� | C�����ܽ⡢�ᾧ���������ķ��� | D����Һ�� |
��1�� ��������Ȼ��ƺʹ���Ļ�����з�����������Ӧ��__________��
��2�����Ҷ����ͱ�������������ѷ�����__________��
���𰸡���1��C��2��A��˵�������¶ȵı仯�Ȼ��ƺʹ�����ܽ�ȵı仯���Ȳ�ͬ���������ܽ⡢�ᾧ���������ķ������룻�Ҷ����ͱ������ķе����ϴ���������
��������
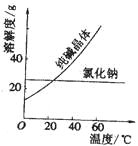
��1�����ݲ���һ���з�����������ܽ�����¶ȵ�Ӱ��Ƚϴ�
��2�����ݲ��϶����з�����������е㲻ͬ����������
��1�����ݲ���һ�������ܽ�����¶ȵ�Ӱ��Ƚϴ�NaCl�ܽ�����¶ȵ�Ӱ��Ƚ�С������ᴿNa2CO3��������ˮ�ܽ����Ƴɱ�����Һ����Һ����ȴ��Na2CO3�����������ܽ⡢�ᾧ�����˵ķ�������ѡ��C��ȷ��
��2�����ݲ��϶������������Ҵ�����������ܣ��������ʵķе����ϴ���˲��������з��룬��ѡ��A��ȷ��

 �п�������㾫��ϵ�д�
�п�������㾫��ϵ�д�����Ŀ��I.����β���dz��е���Ҫ������Ⱦ��о���������β����Ϊ������������Ҫ����������ȼ������ʱ������Ӧ��N2��g��+O2��g��![]() 2NO��g�����÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5 mol N2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
2NO��g�����÷�Ӧ�ǵ�������β���к���NO��ԭ��֮һ��T��ʱ����5L�ܱ������г���6.5 mol N2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5mol��
��1��5 min�ڸ÷�Ӧ��ƽ������v��NO��=___________����T��ʱ���÷�Ӧ��ƽ�ⳣ��ֵΪ_________��
��2����Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯���ǣ�����ţ�___________��
a�����������ܶ� b����������ѹǿ
c������Ӧ���� d����λʱ���ڣ�N2��NO��������֮��
��3����֪������
���� N2(g) + 2O2(g) === 2NO2(g) ��H= + 68 kJ��mol��1
����Ȼ�ѧ����ʽ��˵���¶ȶ���NO����NO2ƽ��ת���ʵ�Ӱ�죺_____________________��
II. ��pm2.5��������Ҫ�ɷ���SO2��NOx��CxHy������������ȡ�
��4����������������������_____________
��5��NaClO2��Һ��������SO2��NO����NaClO2��Һ��ͨ�뺬��SO2��NO�����壬��Ӧ�¶�Ϊ323 K��NaClO2��ҺŨ��Ϊ5��103mol��L1����Ӧһ��ʱ�����Һ������Ũ�ȵķ���������±�
���� | SO42 | SO32 | NO3 | NO2 | Cl |
c/��mol��L1�� | 8.35��104 | 6.87��106 | 1.5��104 | 1.2��105 | 3.4��103 |
��д��NaClO2��Һ������������Ҫ��Ӧ�����ӷ���ʽ________________________
����ѹǿ��NO��ת����______�����ߡ������䡱���͡�����
����ʵ������֪������Ӧ���ʴ���������Ӧ���ʣ���������������С��������ԭ�����SO2�ܽ��Դ���NO����������___________________
��6����ͼ���װ�ÿɽ�SO2��NOת��Ϊ(NH4)2SO4��

�������ĵ缫��Ӧʽ��______________________________
��SO2��NOͨ����װ���е������Ϊ___________________

