��Ŀ����
����ѧ����ѡ��3�����ʽṹ�����ʡ�(15��)
±��Ԫ�صĵ��ʺͻ�����ܶ࣬���ǿ���������ѧ���ʽṹ�����ʵ����֪ʶȥ��ʶ���������ǡ�
��1��±��Ԫ��λ��Ԫ�����ڱ���_________������ļ۵����Ų�ʽΪ____________________��
��2����һ��Ũ�ȵ���Һ�У���������Զ����ӵ�(HF)2��ʽ���ڵġ�ʹ�������ӵϵ���������________��
��3��������±��ṩ�ĵ�һ�����������жϣ����п������ɽ��ȶ��ĵ��������ӵ�±��ԭ����_________��
| | �� | �� | �� | �� |
| ��һ������ (kJ/mol) | 1681 | 1251 | 1140 | 1008 |
��Ƚ϶�������ǿ����HIO3_____ H5IO6(������� ����������)��
��5����֪ClO2�� Ϊ���ͣ�������ԭ����Χ���ĶԼ۲���ӡ�ClO2�� ������ԭ�ӵ��ӻ��������Ϊ___________��д��һ��ClO2�� �ĵȵ�����__________��
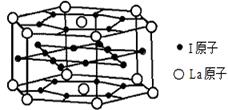
��6����ͼΪ�⾧�徧���ṹ���й�˵������ȷ����_____________��
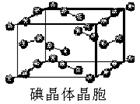
A������ӵ�������2�ֲ�ͬ��ȡ��2��ȡ��ͬ�ĵ������4��λ��������λ�γɲ�ṹ
B���þ�̯����֪ƽ��ÿ����������4����ԭ��
C���⾧��Ϊ��������Ŀռ�ṹ����ԭ�Ӿ���
D���⾧���д��ڵ�������зǼ��Լ��ͷ��»���
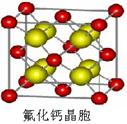
��7����֪CaF2����(��ͼ)���ܶ�Ϊ��g/cm3��NAΪ�����ӵ��������������ڵ�����Ca2���ĺ˼��Ϊa cm����CaF2����Է����������Ա�ʾΪ___________��
��1��P (1��)4S24P5(1��) ��2�� ���(1��)
��3����(2��) ��4����(2��)
��5��sp3 (2��) Cl2O (��OF2�Ⱥ�����) (2��)
��6��AD(2��)
��7��a3��NA/��(2��)
���������������±��Ԫ��λ�����ڱ���17��(P��)����(����Ԫ��)ԭ�ӵļ۵����Ų�Ϊ4S24P5��
���������Ӽ�ͨ������ϳ�(HF)2��
�����ڵ�ĵ�һ��������±��ԭ������Խ�С�����п������ɽ��ȶ��ĵ��������ӡ�
��HIO3�ķ��ǻ���ԭ������2���࣬���Խ�H5IO6 [���ǻ���ԭ����Ϊ1]ǿ��
������ClO2�� ��������ԭ����Χ���ĶԼ۲���ӣ���������ԭ�ӵ��ӻ��������Ϊsp3�����ݡ��������ڡ�ͬ�廥������ԭ���ȷ��ClO2�� �ĵȵ�����ΪCl2O��OF2��
�ʵ⾧�徧���ṹ������������������ÿ�������к���8�� ��6��
��6��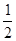 ��4������ӣ�����ԭ����Ϊ8��B����⾧�����ڷ��Ӿ��壬C�����
��4������ӣ�����ԭ����Ϊ8��B����⾧�����ڷ��Ӿ��壬C�����
����CaF2�������Ca2����8�� ��6��
��6��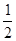 ��4��������F����8�����൱����4��CaF2����ѣ�
��4��������F����8�����൱����4��CaF2����ѣ�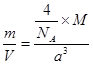 �������M��)a3��NA/����
�������M��)a3��NA/����
���㣺���⿼�����ʽṹ������(Ԫ�صķ������۵����Ų�ʽ���������һ�����ܡ�����������Դ�С����������ؼ������ж�)��

Ԫ�����ڱ���Ԫ����������ѧϰ���о�������ʵ�����к���Ҫ�����á��±��г��ˢ١������Ԫ�������ڱ��е�λ�á�
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | | �� | | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | | | | | | �� | |
��2���ڢ١��ڡ�������Ԫ�ص��������Ӧ��ˮ�����У�������ǿ���� (�ѧʽ)��
��3���١��ڡ�������Ԫ�ذ����Ӱ뾶�ɴ�С��˳������Ϊ (�����ӷ���)��
��4����Ԫ���γɵľ���ǿ�����Ե��⻯��ṹʽ�� �� ��Ԫ����һ���⻯���ڳ�������ڷ�����Ӧ�Ļ�ѧ����ʽΪ ��
��12�֣���ѡ���⡿�������A��B��С�⣬��ѡ������һС�⣬������Ӧ�Ĵ�����������������������AС�����֡�
A��[���ʽṹ������]
�±�ΪԪ�����ڱ���һ���֣����е���ĸ������Ӧ��Ԫ�ء�
| a | | | | | | | | | | | | | | | | | |
| | | | | | | | | | | | | | b | c | d | | |
| | e | | | | | | | | | | | f | | g | | | |
| | | | | | | | | h | I | | | | | | | | |
��2��Ԫ��c��d��e��f�ĵ�һ�����ܣ�I1����С�����˳��Ϊ ��������ӦԪ�ص�Ԫ�ط��ű�ʾ��
��3���������е�Ԫ��֮������γɶ�������������л�������������γɵĻ�����֮һ�����ڼ�����ȩ�ķ�����̼ԭ�ӹ�����ӻ�����Ϊ ��
1 mol ����ȩ�����к��ЦҼ�����ĿΪ ��
��4��Ԫ��d��e�γɵĻ����ﳣ�������� ���ϣ���ԭ���� ��
��5�������й�Ԫ���γɵ�һ�����Ӻ͵���d3��Ϊ�ȵ����壬������ӵĻ�ѧʽΪ ��
��6��Ԫ��I�ĺϽ����������a�ĵ��ʣ��úϽ�ľ����ṹ��ͼ��ʾ����˺Ͻ�Ļ�ѧʽΪ ��
(15��)
A��B��D��E��FΪ������Ԫ�أ��ǽ���Ԫ��A����������������������ͬ��B����������������������������2����B ��D�г��ȼ������������ۻ�����BD2��E����D2��������ͬ�ĵ�������A��F��ȼ�գ���������ˮ�õ�һ��ǿ�ᡣ�ش��������⣺
��1��A�����ڱ��е�λ���� ��д��һ�ֹ�ҵ�Ʊ�����F�����ӷ���ʽ ��
��2��B��D��E��ɵ�һ�����У�E����������Ϊ43%��������Ϊ ����ˮ��Һ��F���ʷ�Ӧ�Ļ�ѧ����ʽΪ ���ڲ����м�������KI����Ӧ�����CC14������ ������ ɫ��
��3������ЩԪ����ɵ����ʣ�����ɺͽṹ��Ϣ���±���
| ���� | ��ɺͽṹ��Ϣ |
| a | ����A�Ķ�Ԫ���ӻ����� |
| b | ���зǼ��Թ��ۼ��Ķ�Ԫ���ӻ������ԭ����֮��Ϊ1:1 |
| c | ��ѧ���ΪBDF2 |
| d | ֻ����һ�������������ҿɵ���ĵ��ʾ��� |
d�ľ��������� ��
��4����A��B��DԪ����ɵ����ֶ�Ԫ�������γ�һ������Դ���ʡ�һ�ֻ��������ͨ�� �����ɾ��п�ǻ�Ĺ��壻��һ�ֻ�����(��������Ҫ�ɷ�)���ӽ���ÿ�ǻ������ӵĿռ�ṹΪ ��
(9��)Ԫ����������ָ������ѧϰԪ�ؼ��仯����֪ʶ����Ҫ���ߡ���֪����Ԫ�أ�����Po���IJ���֪ʶ���±���ʾ��
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵㣨�棩 | -218.4 | 113 | | 450 |
| ���ʷе㣨�棩 | -183 | 444.6 | 685 | 1390 |
| Ԫ����Ҫ���ϼ� | -2 | -2,+4,+6 | -2,+4,+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
��1�����������۵㷶Χ������________________��
��2��Ԫ���ڵ���Ҫ���ϼۿ�����________________��
��3���������ڵ��⻯��ˮ��Һ��������ǿ������˳����________________(�û�ѧʽ��ʾ)��
��4���������н�ǿ��__________��������ԡ���ԭ�ԡ��������¶���ڿ����г��ڱ����ױ��ʣ�����ܷ�����Ӧ�Ļ�ѧ����ʽΪ_________________________________��
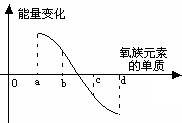
��5����ͼ��ʾΪ����Ԫ�ص�����H2��Ӧ�����е������仯ʾ��ͼ������a��b��c��d�ֱ��ʾ����Ԫ����ijһԪ�صĵ��ʣ�������Ϊ��ͬ���ʵ����ĵ�����H2��Ӧ�����е������仯�������仯��0��ʾ���ȣ������仯��0��ʾ���ȣ�����b����___________ _____�� d���� (��д��������)��
��16�֣��ȵ���ԭ���Ļ����۵��ǣ�ԭ������ͬ�Ҽ۵���������ȵķ��ӻ����Ӿ�����ͬ�Ļ�ѧ�����ͺͿռ乹�ͣ�����Ϊ�ȵ����塣�ȵ�����Ľṹ���ƣ���������������磺N2��CO��C22����CN��Ϊ�ȵ����塣
��1����֪CaC2Ϊ���ӻ������CaC2�ĵ���ʽΪ ��
��2���۱�ϩ���׳�������ë���ɱ�ϩ�����CH2=CH��CN���ۺϷ�Ӧ���ɣ���CH2=CH��CN��Cԭ�ӵ��ӻ���ʽΪ �������ЦҼ��ͦм���֮��Ϊ ��
��3��CO������ɽ���ԭ��M�γ������M(CO)n ��������������ԭ�Ӽ۵���������λ���ṩ��������֮��Ϊ18����MΪFe����n= ��
��4��CO��N2�Ľṹ���ƣ������к��й����������ɱ�ʾΪC��O ���±������ߵļ������ݣ���λ��kJ��mol��1��
| | C��O | C=O | C��O |
| CO | 357.7 | 798.9 | 1071.9 |
| | N��N | N=N | N��N |
| N2 | 154.8 | 418.4 | 941.7 |
��5��Fe3+��Fe2+��Co3+��Co2+������CN-�γ���������������Һ�м������KCN��Һ����������ɫ����K4[Fe(CN)6]������������Һ����ͨ���������������ɫ����K3[Fe(CN)6]����K4[Fe(CN)6]��K3[Fe(CN)6]�����ж������ڵ������������� �������ţ�
��A�����Ӽ����� B�����ۼ����� C������������D����λ������ E�����»���
��6��д����NO3����Ϊ�ȵ�����ķ��� ��д��һ�֣���
(12��)����ѧ�������ʽṹ�����ʡ�
��1�����ɽ���Ԫ�������γɶ��������磺[Fe(H2NCONH2)6](NO3)3[�����������غ���(��)��Fe(CO)x�ȡ�
�ٻ�̬Fe3+��M������Ų�ʽΪ ��
������(H2NCONH2)������Cԭ�ӵ��ӻ���ʽ�� ��
�������Fe(CO)x������ԭ�Ӽ۵������������ṩ������֮��Ϊ18����x= ��
Fe(CO)x�����³�Һ̬���۵�Ϊ-20.5�棬�е�Ϊ103�棬�����ڷǼ����ܼ����ݴ˿��ж�Fe(CO)x�������� (�������)��
��2������˵����ȷ���� (����ĸ���)��
| A����һ�����ܴ�С��S>P>Si |
| B���縺��˳��C<N<O<F |
| C����Ϊ������CaO��KCl�ߣ�����KCl���۵��CaO�۵�� |
| D�����Ӿ����У����ۼ�����Խ�÷��Ӿ�����۷е�Խ�� |
 g��cm-3,�����ӵ�����ΪNA�����߳�a= cm��(�ú�
g��cm-3,�����ӵ�����ΪNA�����߳�a= cm��(�ú� ��NA�ļ���ʽ��ʾ)
��NA�ļ���ʽ��ʾ)