��Ŀ����
(15��)������ˮ��������Ⱦ��������Ҫ���⡣��ˮ�и�Ԫ����Cr2O72-��CrO42-��ʽ���ڣ������������������ǣ�
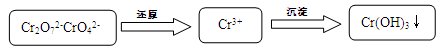
��1���������ʹCrO42-ת��ΪCr2O72-�� 2CrO42- + 2H+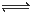 Cr2O72- + H2O
Cr2O72- + H2O
��������pH=1��Һ��Cr2O72-Ũ��Ϊ0��1 mol?L-1��Cr2O72-Ũ����CrO42-Ũ�ȵ�10�����û�ѧƽ�ⳣ��K= ��
��2�����۸��Ķ��Դ�Լ�����۸���100������������ԭ������������Һ��ͨSO2��Cr2O72-��ԭ����Ӧ�����ӷ���ʽΪ �������ټӼCr3+��������֪������Ksp��Cr(OH)3�� =10-32,Ҫʹc(Cr3+)���͵�10-5mol?L-1����Һ��pHӦ���ߵ� ��
��3����ԭ�������õ�������Fe2+��Cr2O72-��ԭΪCr3+�����װ�����õĵ缫������̼������Ƭ��������Ƭ����ֱ����Դ�� ����
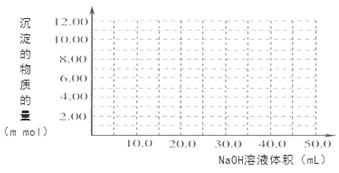
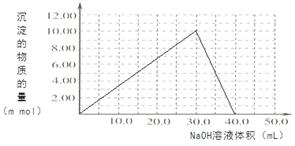
��4��Cr(OH)3Ϊ��ɫճ�Գ�����������Al(OH)3����������������ǿ����Һ��Cr(OH)3����ϡ����Ļ�ѧ����ʽΪ ����100��00mL 0��1000 mol?L-1CrCl3��Һ�μ�1��000mol?L-1NaOH��Һ, �뻭�����ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵͼ:

��1���������ʹCrO42-ת��ΪCr2O72-�� 2CrO42- + 2H+
 Cr2O72- + H2O
Cr2O72- + H2O��������pH=1��Һ��Cr2O72-Ũ��Ϊ0��1 mol?L-1��Cr2O72-Ũ����CrO42-Ũ�ȵ�10�����û�ѧƽ�ⳣ��K= ��
��2�����۸��Ķ��Դ�Լ�����۸���100������������ԭ������������Һ��ͨSO2��Cr2O72-��ԭ����Ӧ�����ӷ���ʽΪ �������ټӼCr3+��������֪������Ksp��Cr(OH)3�� =10-32,Ҫʹc(Cr3+)���͵�10-5mol?L-1����Һ��pHӦ���ߵ� ��
��3����ԭ�������õ�������Fe2+��Cr2O72-��ԭΪCr3+�����װ�����õĵ缫������̼������Ƭ��������Ƭ����ֱ����Դ�� ����
��4��Cr(OH)3Ϊ��ɫճ�Գ�����������Al(OH)3����������������ǿ����Һ��Cr(OH)3����ϡ����Ļ�ѧ����ʽΪ ����100��00mL 0��1000 mol?L-1CrCl3��Һ�μ�1��000mol?L-1NaOH��Һ, �뻭�����ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵͼ:

��1����1��0��105��2��3SO2 + Cr2O72- + 2H+ = 2Cr3+ + 3SO42- + H2O 5
��3�� ����4�� 2Cr(OH)3 + 3H2SO4 = 2Cr2(SO4)3 + 3H2O��
 ��
��
��3�� ����4�� 2Cr(OH)3 + 3H2SO4 = 2Cr2(SO4)3 + 3H2O��
 ��
�������������1����ѧƽ�ⳣ��
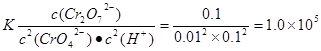 ����2����������Һ��ͨSO2��Cr2O72-��ԭΪCr3+�����ݵ����غ㼰����غ㡢ԭ���غ�ɵ÷�Ӧ�����ӷ���ʽΪ3SO2 + Cr2O72- + 2H+ = 2Cr3+ + 3SO42- + H2O��������Ksp��Cr(OH)3�� =10-32, c(Cr3+)=10-5mol/L,c3(OH-)=10-32��10-5=10-27������c(OH-)=10-9mol/L����c(H+)=Kw��c(OH-)=10-5mol/L,���pH=5����3����ԭ�������õ�������Fe2+��Cr2O72-��ԭΪCr3+�����װ�����õĵ缫������̼������Ƭ��������Ƭ��Ҫʧȥ���ӱ�ΪFe2+������ƬҪ���Դ���������ӡ�Cr(OH)3��ϡ���ᷴӦ��ѧ����ʽΪ2Cr(OH)3 + 3H2SO4 = 2Cr2(SO4)3 + 3H2O��n(Cr3+)=0��1L��0��100mol/L=0��01mol�������Һ�м���NaOH���ȷ�����Ӧ��Cr3����3OH-��Cr(OH)3����n(NaOH)= 0��03mol����NaOH�����Ϊ30mlʱ�����ﵽ���ֵ���ټ���NaOH��Һ��������Ӧ��Cr(OH)3+ OH-= CrO2-+ 2H2O����ʱ������NaOH�����ʵ���Ϊ0��01mol,��������10ml�����ﵽ40mlʱ��������ȫ�ܽ⡣֮���ٵμ�NaOH��Һ��Ҳ���ᷢ����Ӧ�����ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵͼΪ
����2����������Һ��ͨSO2��Cr2O72-��ԭΪCr3+�����ݵ����غ㼰����غ㡢ԭ���غ�ɵ÷�Ӧ�����ӷ���ʽΪ3SO2 + Cr2O72- + 2H+ = 2Cr3+ + 3SO42- + H2O��������Ksp��Cr(OH)3�� =10-32, c(Cr3+)=10-5mol/L,c3(OH-)=10-32��10-5=10-27������c(OH-)=10-9mol/L����c(H+)=Kw��c(OH-)=10-5mol/L,���pH=5����3����ԭ�������õ�������Fe2+��Cr2O72-��ԭΪCr3+�����װ�����õĵ缫������̼������Ƭ��������Ƭ��Ҫʧȥ���ӱ�ΪFe2+������ƬҪ���Դ���������ӡ�Cr(OH)3��ϡ���ᷴӦ��ѧ����ʽΪ2Cr(OH)3 + 3H2SO4 = 2Cr2(SO4)3 + 3H2O��n(Cr3+)=0��1L��0��100mol/L=0��01mol�������Һ�м���NaOH���ȷ�����Ӧ��Cr3����3OH-��Cr(OH)3����n(NaOH)= 0��03mol����NaOH�����Ϊ30mlʱ�����ﵽ���ֵ���ټ���NaOH��Һ��������Ӧ��Cr(OH)3+ OH-= CrO2-+ 2H2O����ʱ������NaOH�����ʵ���Ϊ0��01mol,��������10ml�����ﵽ40mlʱ��������ȫ�ܽ⡣֮���ٵμ�NaOH��Һ��Ҳ���ᷢ����Ӧ�����ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵͼΪ ��
��
��ϰ��ϵ�д�
�����Ŀ
 CH3OH��g����H1����116 kJ��mol��1
CH3OH��g����H1����116 kJ��mol��1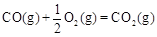 ��H2����283 kJ��mol��1
��H2����283 kJ��mol��1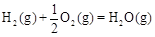 ��H3����242 kJ��mol��1
��H3����242 kJ��mol��1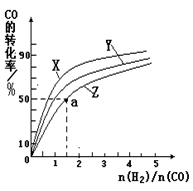
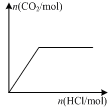
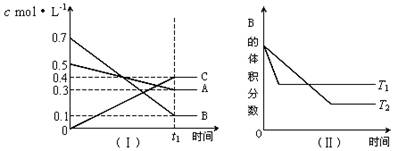
 ��2(t��BuNO) �����¶��¸÷�Ӧ��CCl4�ܼ��е�ƽ�ⳣ��Ϊ1.4��
��2(t��BuNO) �����¶��¸÷�Ӧ��CCl4�ܼ��е�ƽ�ⳣ��Ϊ1.4�� 
 2SO3��g�� ��H��0��2min��Ӧ�ﵽƽ�⣬����SO3Ϊ1.4mol,ͬʱ�ų�����Q kJ�������з�����ȷ����
2SO3��g�� ��H��0��2min��Ӧ�ﵽƽ�⣬����SO3Ϊ1.4mol,ͬʱ�ų�����Q kJ�������з�����ȷ���� 2NH3(g)+ CO2(g)������˵���÷ֽⷴӦ�ﵽƽ��״̬���� �� ��
2NH3(g)+ CO2(g)������˵���÷ֽⷴӦ�ﵽƽ��״̬���� �� ��

 CH3OH��g��+H2O��g����H<0������6mo1 CO2��8mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���
CH3OH��g��+H2O��g����H<0������6mo1 CO2��8mol H2����2L���ܱ������У����H2�����ʵ�����ʱ��仯����������ͼ��ʾ��ʵ�ߣ���

 cP(g)��dQ(g)�ﵽƽ��ʱ��M���������y(M)�뷴Ӧ�����Ĺ�ϵ��ͼ��ʾ������z��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȣ�����˵����ȷ����(����)
cP(g)��dQ(g)�ﵽƽ��ʱ��M���������y(M)�뷴Ӧ�����Ĺ�ϵ��ͼ��ʾ������z��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȣ�����˵����ȷ����(����)