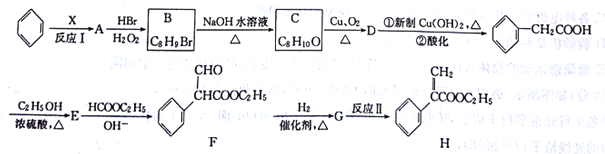
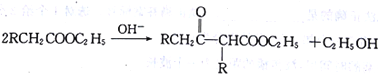��Ŀ����
����Ŀ��������(V)���仯�������Ź㷺����;����ش���������:
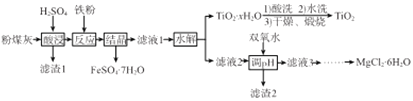
��1��������Һ�е���Ҫ�ۺ�״̬����Һ��pH��ϵ��ͼ1��ʾ��V2O74-��VԪ�صĻ��ϼ���_____����д����Һ��VO3-ת��ΪV2O74-�����ӷ���ʽ:____________��
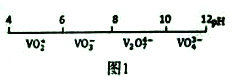
��2�����������γ�������ԭ�����ں��з�Ԫ�ص���Һ�м�����κ��γ�NH4VO3������ͼ2���ڹ�ҵ�����в�ͬpH�����³����ʵIJⶨֵ��ʵ�ʹ�ҵ�����г�ѡ��pH=7.5Ϊ�����������������pH����8.0ʱ�����ʽ��ͣ���ԭ������Һ��VO3-ת��ΪV2O74-��_______��(����д��һ��ԭ��)
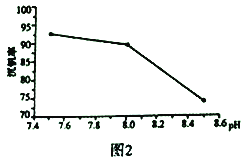
��3��NH4VO3�ڸ����·ֽ������V2O5����Ϊ���Ṥҵ��2SO2(g)+O2(g)![]() 2SO3(g) ��H=p�Ĵ��������ԭ����ͼ3��ʾ��
2SO3(g) ��H=p�Ĵ��������ԭ����ͼ3��ʾ��
������a����b�Ļ�ѧ����ʽΪ:V2O5(s)+SO2(g)=V2O4(s)+SO3(g)��H=q��
V2O4(s)+O2(g)+2SO2(g)=2VOSO4(g)��H=r
��д������c���Ȼ�ѧ����ʽ:_________________��
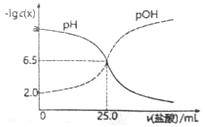
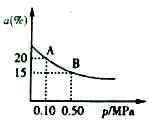
��t2���£���Ӧ:2SO3(g)![]() 2SO2(g)+O2(g)��H>0��SO3��ƽ��ת����(a)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��t���£���2molSO3����10L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa��B��Ļ�ѧƽ�ⳣ����ֵ��__________��
2SO2(g)+O2(g)��H>0��SO3��ƽ��ת����(a)����ϵ��ѹǿ(p)�Ĺ�ϵ��ͼ��ʾ��t���£���2molSO3����10L�ܱ������У���Ӧ��ƽ�����ϵ��ѹǿΪ0.10MPa��B��Ļ�ѧƽ�ⳣ����ֵ��__________��
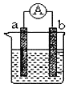
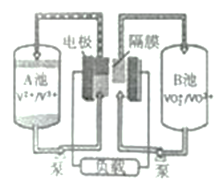
(4)ȫ��ҹ�������һ�ֿɳ���أ�װ����ͼ��ʾ�����ڷŵ��������H+��A������B�أ���:
���ŵ�����У������õ���_______��(����A������B��)��
���������У���������ʽΪ____________��
���𰸡� +5 2 VO3- + 2OH- ![]() V2O74- + H2O pH������Һ�е�NH4+��ת��ΪNH3��H2O 2VOSO4(s)=V2O5(s)+SO3(g)+SO2(g) ��H=p-q-r 0.00125 A�� VO2+ - e- +H2O = VO2+ + 2H+
V2O74- + H2O pH������Һ�е�NH4+��ת��ΪNH3��H2O 2VOSO4(s)=V2O5(s)+SO3(g)+SO2(g) ��H=p-q-r 0.00125 A�� VO2+ - e- +H2O = VO2+ + 2H+
�������������������1�����������л��ϼ۴����͵�������������ɼ���Ԫ�ػ��ϼ�������ͼʾ���ڼ�����Һ��VO3-ת��ΪV2O74-����2����pH����8.0ʱ����Һ��VO3-ת��ΪV2O74-����Һ�е�NH4+��ת��ΪNH3��H2O�����Ե��³����ʽ�������3�������ݸ�˹���ɼ������c���Ȼ�ѧ�����������á�����ʽ������ƽ�ⳣ����(4)�ŵ��������H+��A������B�أ�˵��A�Ǹ���![]() ��B������VO2+ + 2H++ e- = VO2+ +H2O������������������������ӵ�Դ������������������
��B������VO2+ + 2H++ e- = VO2+ +H2O������������������������ӵ�Դ������������������
��������V2O74-��VԪ�صĻ��ϼ���x����2x+(-2) ![]() 7=-4��x=+5������V2O74-��VԪ�صĻ��ϼ���+5������ͼʾ���ڼ�����Һ��VO3-ת��ΪV2O74-��ת�������ӷ���ʽ��2 VO3- + 2OH-
7=-4��x=+5������V2O74-��VԪ�صĻ��ϼ���+5������ͼʾ���ڼ�����Һ��VO3-ת��ΪV2O74-��ת�������ӷ���ʽ��2 VO3- + 2OH- ![]() V2O74- + H2O����2����pH����8.0ʱ����Һ��VO3-ת��ΪV2O74-����Һ�е�NH4+��ת��ΪNH3��H2O�����Ե��³����ʽ�������3����2SO2(g)+O2(g)
V2O74- + H2O����2����pH����8.0ʱ����Һ��VO3-ת��ΪV2O74-����Һ�е�NH4+��ת��ΪNH3��H2O�����Ե��³����ʽ�������3����2SO2(g)+O2(g)![]() 2SO3(g) ��H=p ����V2O5(s)+SO2(g)=V2O4(s)+SO3(g)��H=q����V2O4(s)+O2(g)+2SO2(g)=2VOSO4(g)��H=r�����ݸ�˹��������-��-�۵�2VOSO4(s)=V2O5(s)+SO3(g)+SO2(g) ��H=p-q-r ��
2SO3(g) ��H=p ����V2O5(s)+SO2(g)=V2O4(s)+SO3(g)��H=q����V2O4(s)+O2(g)+2SO2(g)=2VOSO4(g)��H=r�����ݸ�˹��������-��-�۵�2VOSO4(s)=V2O5(s)+SO3(g)+SO2(g) ��H=p-q-r ��
����A���

A���ƽ�ⳣ��![]() ��A��B�¶���ͬ��ƽ�ⳣ����ͬ������B���ƽ�ⳣ����0.00125��(4)���ݷŵ��������H+��A������B�أ�˵��A�Ǹ�����B���������ٷŵ�����У������õ���A�أ��ڳ�������������������ӵ�Դ������������������������ӦΪVO2+ - e- +H2O = VO2+ + 2H+��
��A��B�¶���ͬ��ƽ�ⳣ����ͬ������B���ƽ�ⳣ����0.00125��(4)���ݷŵ��������H+��A������B�أ�˵��A�Ǹ�����B���������ٷŵ�����У������õ���A�أ��ڳ�������������������ӵ�Դ������������������������ӦΪVO2+ - e- +H2O = VO2+ + 2H+��