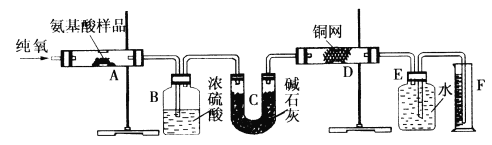
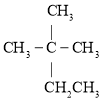
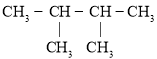��Ŀ����
����Ŀ����������Ҫ�Ļ�������ԭ�ϣ���ҵ�ϳ���������������SO2��SO2������SO3������98.3�����ҵ�Ũ��������SO3�õ������������ᣨH2SO4��SO3����������Ƶõ����������������Ƹ��ֲ�ͬŨ�ȵ��������ڹ�ҵ������
������м��㣺
��1��1kg98%��Ũ��������SO3������___kg�����������ᡣ
��2�������������ᣨH2SO4��SO3������ˮ������SO3��ת��Ϊ���ᡣ����890g����������������ˮ���4.00L���ᣬ����������ʵ���Ũ��Ϊ___mol/L��
��3���������������յĻ�ѧ��Ӧ���£�3FeS2��8O2��Fe3O4��6SO24FeS2��11O2��2Fe2O3��8SO2
��1�ֺ�FeS280���������������Ͽ��������ٶ�98%��Ũ����__?
����24molFeS2��ȫ��Ӧ��������1467.2L����״���������㷴Ӧ������Fe3O4��Fe2O3���ʵ���֮��___��
��4����������ȡ���ᣬ���ܳ��������Դ���ܱ�����������һ�ֺ��з�չǰ;���Ʊ�����ķ�����������ˮ�����Ļ�������ڿ�������ȫȼ�գ��پ�����������ȴ�Ƶ���98%��Ũ���ᣨ����������SO2��ʧ2%��������ˮ����ʧˮ���������ڻ�����е��������___��
���𰸡�1.978kg 2.5mol/L 1.33�� 2��9 0.918
��������
��1��1kg 98%��Ũ������H2SO4������Ϊ1kg��98%=0.98g����H2O������Ϊ1kg-0.98g=0.02kg����H2O+SO3=H2SO4����֪����H2SO4������Ϊ0.02kg��![]() ����H2SO4������=0.98g+0.02kg��
����H2SO4������=0.98g+0.02kg��![]() =
=![]() kg�����ݹ�ϵʽH2SO4��H2SO4SO3����֪�õ�������������Ϊ��
kg�����ݹ�ϵʽH2SO4��H2SO4SO3����֪�õ�������������Ϊ��![]() kg��
kg��![]() =1.978kg���ʴ�Ϊ��1.978��
=1.978kg���ʴ�Ϊ��1.978��
��2��H2SO4SO3�����ʵ���Ϊ![]() =5mol��������Ԫ���غ�n��H2SO4��=2n��H2SO4SO3��=10mol������������ʵ���Ũ��Ϊ
=5mol��������Ԫ���غ�n��H2SO4��=2n��H2SO4SO3��=10mol������������ʵ���Ũ��Ϊ![]() =2.5mol/L���ʴ�Ϊ��2.5��
=2.5mol/L���ʴ�Ϊ��2.5��
��3�����������Ͽ�����x��98%��Ũ���ᣬ��
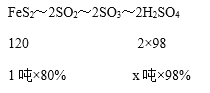
����120��2��98=1����80%��x����98%�����x=1.33�������Ͽ�����1.33��98%��Ũ���
�ڸ�����Ԫ���غ㣬n��SO2��=2n��FeS2��=48mol�������������ʵ���Ϊ65.5mol����Fe3O4��Fe2O3���ʵ����ֱ�Ϊxmol��
ymol������Feԭ���غ㡢Oԭ���غ㣬��
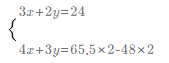 ��ã�
��ã�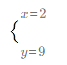 ����Fe3O4��Fe2O3���ʵ���֮��Ϊ2��9���𣺷�Ӧ������Fe3O4��Fe2O3���ʵ���֮��Ϊ2��9��
����Fe3O4��Fe2O3���ʵ���֮��Ϊ2��9���𣺷�Ӧ������Fe3O4��Fe2O3���ʵ���֮��Ϊ2��9��
��4��98%��Ũ������H2SO4��H2O�����ʵ���֮��Ϊ![]() ��98%��Ũ���ữѧʽ���Ա�ʾΪH2SO4��
��98%��Ũ���ữѧʽ���Ա�ʾΪH2SO4��![]() H2O����������Ϊamol������������SO2��ʧ2%����SԪ��������Ϊ98%������SԪ���غ㣬H2SO4��
H2O����������Ϊamol������������SO2��ʧ2%����SԪ��������Ϊ98%������SԪ���غ㣬H2SO4��![]() H2O�����ʵ���amol��98%������Hԭ���غ㣬������ˮ���������ʵ���Ϊamol��98%����1+
H2O�����ʵ���amol��98%������Hԭ���غ㣬������ˮ���������ʵ���Ϊamol��98%����1+![]() ��=
��=![]() ����������������Ϊ
����������������Ϊ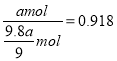 ����0.918��
����0.918��

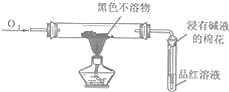
����Ŀ����ͼ��ijͬѧ�о�ͭ��Ũ����ķ�Ӧװ�ã�
���������գ�
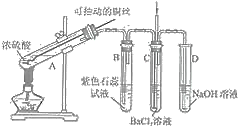
(1)д��A�з�����Ӧ�Ļ�ѧ����ʽ______�����ÿɳ鶯ͭ˿����ʵ����ŵ���______��
(2)��Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ______��
(3)��C�Թܵ�ֱ����������BaCl2��Һ��ͨ����һ�����壬������ɫ�����������������______��______����Ҫ����һ�ֻ������һ�ֵ��ʵĻ�ѧʽ����
(4)��Ӧ��ϣ���A�еĻ���ﵹ��ˮ�У��õ������Ե���ɫ��Һ��������ɫ�����������ò�����IJ�����______���ú�ɫ�����ﲻ������CuO��������______��
(5)��ͭ��Ũ���ᷴӦ�����ĺ�ɫ���������̽����ʵ��װ�ü��������£�
ʵ��װ�� | ʵ������ |
| Ʒ����ɫ |
�ٸ���ʵ��������жϺ�ɫ��������һ������______Ԫ�ء�
�����ú�ɫ������������Ԫ��������ڷ�Ӧǰ���������ֲ��䣬�Ʋ��仯ѧʽΪ______��