��Ŀ����
CO�����ںϳɼ״���һ���¶��£������Ϊ2L���ܱ������м���CO��H2��������ӦCO��g��+2H2��g��?CH3OH��g������ƽ����ø����Ũ�����£�
�ٻ�������ƽ����Է�������______��
����ʽ������ƽ�ⳣ��K=______��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c��H2����ȡֵ��Χ��______��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������
| ���� | CO | H2 | CH3OH |
| Ũ�ȣ�mol?L-1�� | 0.9 | 1.0 | 0.6 |
����ʽ������ƽ�ⳣ��K=______��
�������������ѹ��Ϊ1L���������㣬Ԥ����ƽ����c��H2����ȡֵ��Χ��______��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH����ʱv��______v�������������������=������
��ͼ�����ݷ�����֪��n��CO��=2L��0.9mol/L=1.8mol��n��H2��=2L��1.0mol/L=2mol��n��CH3OH��=2L��0.6mol/L=1.2mol��
��������ƽ��Ħ������=
=
=18.56g/mol��
���Ի�������ƽ����Է�������18.56��
�ʴ�Ϊ��18.56��
��CO��g��+2H2��g��?CH3OH��g������ͼ��������ƽ��Ũ�ȿ�֪��K=
=0.67L2?moL-2��
�ʴ�Ϊ��
=0.67L2?moL-2��
��CO��g��+2H2��g��?CH3OH��g���������������ѹ��Ϊ1L��������Ũ��Ӧ��Ϊԭ����2������ѹǿ����ƽ�������������С�ķ�����У���Ӧ������У�ƽ��Ũ��С��2mol/L������������ƽ��Ũ��Ӧ1mol?L-1��c��H2����2mol?L-1��
�ʴ�Ϊ��1mol?L-1��c��H2����2mol?L-1��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH���� ������Ũ��Ϊ
CO��g��+2H2��g��?CH3OH��g����
0.9+0.6=1.5 1.0 0.6+0.4=1
Q=
=0.67=K
˵�����ôﵽ��ƽ���ԭ����ƽ��״̬��ͬ����V��=V����
�ʴ�Ϊ��=��
��������ƽ��Ħ������=
| ������ |
| �����ʵ��� |
| 1.8mol��28g/mol+2mol��28g/mol+1.2mol��2g/mol |
| 1.8mol+2mol+1.2mol |
���Ի�������ƽ����Է�������18.56��
�ʴ�Ϊ��18.56��
��CO��g��+2H2��g��?CH3OH��g������ͼ��������ƽ��Ũ�ȿ�֪��K=
| 0.6 |
| 0.9��1��02 |
�ʴ�Ϊ��
| 0.6 |
| 0.9��1��02 |
��CO��g��+2H2��g��?CH3OH��g���������������ѹ��Ϊ1L��������Ũ��Ӧ��Ϊԭ����2������ѹǿ����ƽ�������������С�ķ�����У���Ӧ������У�ƽ��Ũ��С��2mol/L������������ƽ��Ũ��Ӧ1mol?L-1��c��H2����2mol?L-1��
�ʴ�Ϊ��1mol?L-1��c��H2����2mol?L-1��
��������������䣬�ٳ���0.6molCO��0.4molCH3OH���� ������Ũ��Ϊ
CO��g��+2H2��g��?CH3OH��g����
0.9+0.6=1.5 1.0 0.6+0.4=1
Q=
| 1 |
| 1.5��12 |
˵�����ôﵽ��ƽ���ԭ����ƽ��״̬��ͬ����V��=V����
�ʴ�Ϊ��=��

��ϰ��ϵ�д�
 �����Ļ���������人������ϵ�д�
�����Ļ���������人������ϵ�д� ���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д�
���������ּ���ÿһ��ȫ�º�����ҵ��ϵ�д� ��ٽ������½������������ϵ�д�
��ٽ������½������������ϵ�д�
�����Ŀ
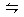 p C(g)���ﵽƽ��������������ѹ����ԭ����һ�룬���ٴ�ƽ��ʱC����Һ��ԭ����1.9����������������ȷ���� ( )
p C(g)���ﵽƽ��������������ѹ����ԭ����һ�룬���ٴ�ƽ��ʱC����Һ��ԭ����1.9����������������ȷ���� ( )