��Ŀ����
Q��W��X��Y��ZΪ����ԭ�����������Ķ�����Ԫ�ء�
��֪����Qԭ�Ӻ�����������ڵ��Ӳ���������W��ɵĻ���������������Ҫ�ɷ֣�
��W��Y��X��Y��ɵĻ������ǻ������ų��Ĵ�����Ⱦ�
��Y��Z���γɵ�������Ϊ30��38���������ӻ����
��1��W�����ڱ���λ�ã�________��������Ϊ38��Y��Z�γɻ�����ĵ���ʽ��________��
��2����ҵ�ϳ�XQ3����H��0�����д�ʩ���ܼӿ췴Ӧ���ʣ�����ʹԭ��ת����һ������ߵ���________��
| A�������¶� |
| B����������� |
| C����XQ3��ʱ�����ȥ |
| D������Ӧ��ϵѹǿ |
��3����״����22.4 L X����̬�⻯���0.5 L 1 mol/L��X������������ˮ���ﷴӦ�����ü�����Һ������Ũ���ɴ�С˳����_______���û�ѧʽ��ʾ����
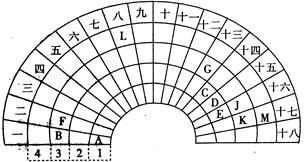
��1���ڶ����ڢ�A�塡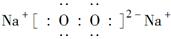
��2��D����3��c��NH4+����c��NO3-����c��OH������c��H����
����

��ϰ��ϵ�д�
 �Ƹ�С״Ԫͬ������������ϵ�д�
�Ƹ�С״Ԫͬ������������ϵ�д�
�����Ŀ
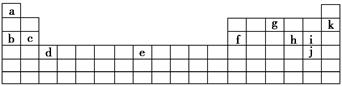
������Ԫ�����ڱ���һ���֣�����Ԫ�آ٣����ڱ��е�λ�ã����û�ѧ����ش��������⣺
| �� ���� | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | | �� | |
��1���ܡ��ݡ���ԭ�Ӱ뾶�ɴ�С��˳��Ϊ(Ԫ�ط���)________________________��
��2���ڡ��ۡ��ߵ���ۺ������������ǿ������˳����(�ѧʽ)________ ��
��3���١��ܡ��ݡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����Թ��ۼ��Ļ����д������һ�ֻ�����Ļ�ѧʽ��_______________��
��4���ɢں͢���ɵĻ�������ݵ�ͬ������������Ԫ�صĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ:_______��
��5��������ݵ�����������ˮ���ﷴӦ�����ӷ���ʽΪ ��
��6�����â٢��������л�����Ϊȼ�ϵ�ص�ԭ�ϣ���д���ڼ��Խ�����ȼ�ϵ�ظ����ĵ缫��Ӧʽ: ��
��7��ȼú�����еĺ��е������NOx����������̼�����壬�������з�����ȼú����������������ʱ�������ü������ԭ�������
�磺CH4(g)��4NO2(g)=4NO(g)��CO2(g)��2H2O(g) �� ��H=��574 kJ��mol��1
CH4(g)��4NO(g)=2N2(g)��CO2(g)��2H2O(g) �� ��H=��1160 kJ��mol��1
��CH4(g)��NO2(g)��ԭΪN2(g)�ȵ��Ȼ�ѧ����ʽΪ ��
�±�Ϊ���ֶ�����Ԫ�ػ��ϼۼ���Ӧԭ�Ӱ뾶�����ݣ�
| Ԫ������ | Ԫ�ر�� | |||||||
| A | B | C | D | E | F | G | H | |
| ԭ�Ӱ뾶(nm) | 0.102 | 0.110 | 0.117 | 0.074 | 0.075 | 0.071 | 0.099 | 0.077 |
| ����ϼ� | ��6 | ��5 | ��4 | | ��5 | | ��7 | ��4 |
| ��ͻ��ϼ� | ��2 | ��3 | ��4 | ��2 | ��3 | ��1 | ��1 | ��4 |
��֪��
��A��D���γɻ�����AD2��AD3��
��E��D���γɶ��ֻ��������ED��ED2�dz����Ļ����C�������ƹ��ء�
(1)E�����ڱ���λ����________________��
(2)C��H����̬�⻯����ȶ���ǿ����ϵΪ______________(�÷���ʽ��ʾ)��
(3)�������ΪADG2��������ˮ�л�ǿ��ˮ�⣬����ʹƷ����Һ��ɫ����ɫ�����һ��ǿ�ᡣ�÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________��
(4)��ҵ�Ͽ��ô�����Һ����ED��ED2���÷�Ӧ���£�
ED��ED2��Na2CO3===2________��CO2
������ij�εĻ�ѧʽӦΪ________��
(5)��һ�ܱ������з�����Ӧ2AD2��D2
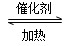 2AD3����H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ���________(����ӡ������١����䡱)��ԭ��Ϊ______________________________________________
2AD3����H����47 kJ/mol��������ƽ����ϵ�м���18D2����ƽ�ⷢ���ƶ���AD2��18D�İٷֺ���________(����ӡ������١����䡱)��ԭ��Ϊ______________________________________________(6)�����һ��ʵ�鷽����ʹͭ��ϡ��H2AD4��Һ��Ӧ���õ���ɫ��Һ�������������з����ڻ����ʵ�鷽��װ��ͼ��