��Ŀ����
��16�֣���ҵ�ϳ�������Ȼ�����������أ�������������ͼ��ʾ��
�ش��������⣺
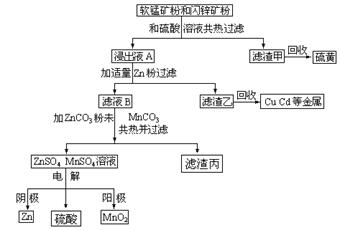
��1��������2������Ҫ�ɷ��� ��
��2����ĸҺ�����ѭ��ʹ�û���ɲ�Ʒ���Ƚ��ͣ������ķ����ǣ��ڶ��ѭ����ġ�ĸҺ���м���NaOH��Һ���䷴Ӧԭ����
�������ӷ�Ӧ����ʽ��ʾ��
��3������Һ1����Ũ�������У��ɼ���������KCl���壬��߲�Ʒ�IJ�����ԭ���� ��
��4����Ҫ��һ���������ؾ���Ĵ��ȣ���������еIJ�����:
��

�ش��������⣺
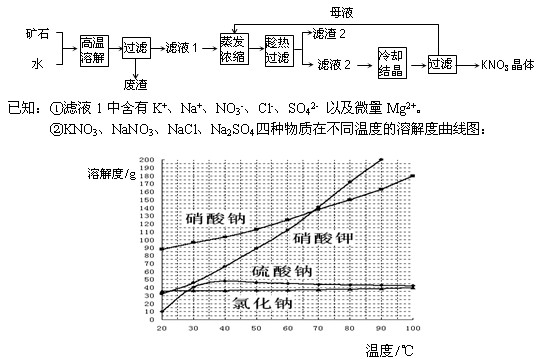
��1��������2������Ҫ�ɷ��� ��
��2����ĸҺ�����ѭ��ʹ�û���ɲ�Ʒ���Ƚ��ͣ������ķ����ǣ��ڶ��ѭ����ġ�ĸҺ���м���NaOH��Һ���䷴Ӧԭ����
�������ӷ�Ӧ����ʽ��ʾ��
��3������Һ1����Ũ�������У��ɼ���������KCl���壬��߲�Ʒ�IJ�����ԭ���� ��
��4����Ҫ��һ���������ؾ���Ĵ��ȣ���������еIJ�����:
��
��1��NaCl��Na2SO4��
��2�� Mg2++2OH-="==" Mg��OH��2��
��3��������Һ��K����Ũ�ȣ�ʹ��Һ��NO3-�����ת��ΪKNO3������
��4���ؽᾧ
��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
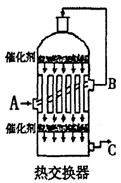
 2ZnO��2SO2 2C��O2
2ZnO��2SO2 2C��O2