��Ŀ����
10��NA��ʾ�����ӵ�����������˵������ȷ���ǣ���������46g NO2��N2O4�Ļ�������к��е�ԭ�Ӹ���Ϊ3NA
�ڳ����£�4g CH4����NA��C-H���ۼ�
��10mL��������Ϊ98%��H2SO4����ˮ��100mL��H2SO4����������Ϊ9.8%
�ܱ�״���£�5.6L SO3���еķ�����Ϊ0.25NA
��25��ʱ��pH=12��1.0L NaClO��Һ��ˮ�������OH-����ĿΪ0.01NA
��0.1mol•L-1Na2CO3��Һ�к���CO32-��ĿС��0.1NA
��1mol Na2O2��ˮ��ȫ��Ӧʱת�Ƶ�����Ϊ2NA��
| A�� | �ۢޢ� | B�� | �٢ڢ� | C�� | �ڢܢ� | D�� | �٢ڢܢ� |
���� ��NO2��N2O4�����ʽ��ͬΪNO2������46g NO2������ԭ������
�������������ʵ�����Ȼ�����1mol�����к�4molC-H����������
����������Ũ��Խ��������ܶ�Խ����10mL��������Ϊ98%��H2SO4����ˮ��100mL��H2SO4��������������9.8%��
�ܱ�״���£���������Ϊ���壬����ʹ�ñ���µ�����Ħ���������������ʵ�����
�ݴ���������Һ�е�������������ˮ����ģ��ݴ˼����1L����Һ�к��е�������������Ŀ��
������������Ԫ��Ϊ-1�ۣ�1mol Na2O2��ˮ��ȫ��Ӧ����0.5mol������ת����1mol���ӣ�
��2Na2O2+2H2O=4NaOH+O2����ÿ����1molO2ʱ��Ӧ��ת�Ƶĵ���Ϊ2mol��
��� �⣺��NO2��N2O4�����ʽ��ͬΪNO2������46g NO2������ԭ����=$\frac{46g}{46g/mol}$��3��NA=3NA���ʢ���ȷ��
��4g CH4���ʵ���Ϊ0.25mol�������к�C-H���ۼ�4��0.25��NA=NA�����ʢ���ȷ��
��10mL��������Ϊ98%��H2SO4����ˮϡ����100mL������������Һ���ܶȴ���ˮ���ܶȣ�����ϡ�ͺ�H2SO4��������������9.8%���ʢ۴���
�ܱ�״���£�5.6L������������ʵ�������0.25mol���ʢܴ���
��25��ʱ��pH=12��1.0L NaClO��Һ�����������ӵ����ʵ���Ϊ0.01mol����Һ��������������ˮ����ģ�����ˮ�������OH-����ĿΪ0.01NA���ʢ���ȷ��
����Һ�������ȷ���������̼����ĸ������ʢ���
��2Na2O2+2H2O=4NaOH+O2����ÿ����1molO2ʱ2molNa2O2��ˮ�귴Ӧ��ת�Ƶĵ���Ϊ2NA���ʢߴ���
��ѡB��
���� ���⿼���˰���٤���������йؼ��㣬�������չ�ʽ��ʹ�ú����ʵĽṹ�ǽ���ؼ����ѶȲ���

 53���ò�ϵ�д�
53���ò�ϵ�д�| A�� | BOH����ˮ������뷽��ʽ��BOH�TB++OH- | |
| B�� | ����һ������������Һ��Ϻ�pH=7����c��A- ��=c��B+�� | |
| C�� | ��0.1mol/L BA��Һ�У�c��B+����c��A- ����c��OH- ����c��H+�� | |
| D�� | ����0.1mol/L BOH��Һϡ����0.001mol/L������Һ��pH=9 |
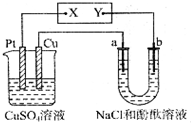
| A�� | X�Ǹ�����Y������ | B�� | Pt��������Cu������ | ||
| C�� | CuSO4��Һ��pH��С | D�� | CuSO4��Һ��pH���� |

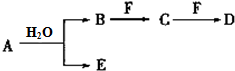 A��B��C��D��E��FΪ��ѧ��ѧ�еij������ʣ�������A��1��2�ֶ�����Ԫ����ɣ���һ��������������ת����ϵ��������������⣺
A��B��C��D��E��FΪ��ѧ��ѧ�еij������ʣ�������A��1��2�ֶ�����Ԫ����ɣ���һ��������������ת����ϵ��������������⣺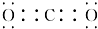 ��D��������ѧ�����������Ӽ������ۼ���
��D��������ѧ�����������Ӽ������ۼ���
