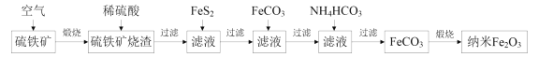
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ―«¬»ΥαΡΤΘ®NaClO2Θ© «“Μ÷÷ΗΏ–ßΒΡΤ·ΑΉΦΝΚΆ―θΜ·ΦΝΘ§Ω…”Ο”ΎΗς÷÷œΥΈ§ΚΆΡ≥–© ≥ΤΖΒΡΤ·ΑΉΓΘ¬μΒΌ―ΖΘ®MathiesonΘ©Ζ®÷Τ±Η―«¬»ΥαΡΤΒΡΝς≥Χ»γœ¬ΘΚ
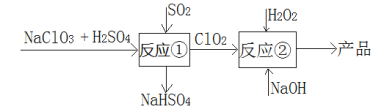
œ¬Ν–ΥΒΖ®¥μΈσΒΡ «Θ® Θ©
A.Ζ¥”ΠΔΌΫΉΕΈΘ§≤ΈΦ”Ζ¥”ΠΒΡNaClO3ΚΆSO2ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ2ΘΚ1
B.»τΖ¥”ΠΔΌΆ®Ιΐ‘≠Βγ≥Ίά¥ Βœ÷Θ§‘ρClO2 «’ΐΦΪ≤ζΈο
C.Ζ¥”ΠΔΎ÷–ΒΡH2O2Ω…”ΟNaClO4¥ζΧφ
D.Ζ¥”ΠΔΎΧθΦΰœ¬Θ§ClO2ΒΡ―θΜ·–‘¥σ”ΎH2O2
ΓΨ¥πΑΗΓΩC
ΓΨΫβΈωΓΩ
AΘ°ΗυΨίΝς≥ΧΆΦΖ¥”ΠΔΌ÷–―θΜ·ΦΝ «NaClO3Θ§ΜΙ‘≠ΦΝ «SO2Θ§ΜΙ‘≠≤ζΈο «ClO2Θ§―θΜ·≤ζΈο «NaHSO4Θ§ΗυΨίΜ·ΚœΦέ…ΐΫΒœύΒ»Ω…ΒΟNaClO3ΚΆSO2ΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣ2:1Θ§Aœν’ΐ»ΖΘΜ
BΘ°”…Ζ¥”ΠΔΌΜ·ΚœΦέ±δΜ·«ιΩωΘ§‘ΌΗυΨί‘≠Βγ≥Ί’ΐΦΪ±μΟφΖΔ…ζΜΙ‘≠Ζ¥”ΠΘ§Υυ“‘ClO2 «’ΐΦΪ≤ζΈοΘ§Bœν’ΐ»ΖΘΜ
CΘ°ΨίΝς≥ΧΆΦΖ¥”ΠΔΎΘ§‘ΎClO2”κH2O2ΒΡΖ¥”Π÷–Θ§ClO2ΉΣΜ·ΈΣNaClO2¬»‘ΣΥΊΒΡΜ·ΚœΦέΫΒΒΆΘ§Ήω―θΜ·ΦΝΘΜH2O2÷ΜΡήΉωΜΙ‘≠ΦΝΘ§―θ‘ΣΥΊΒΡΜ·ΚœΦέ…ΐΗΏΘ§≤ΜΡή”ΟNaClO4¥ζΧφH2O2Θ§Cœν¥μΈσΘΜ
DΘ°ΨίΝς≥ΧΆΦΖ¥”ΠΔΎClO2”κH2O2Ζ¥”ΠΒΡ±δΦέ«ιΩωΘ§ClO2Ήω―θΜ·ΦΝΘ§H2O2ΉωΜΙ‘≠ΦΝΘ§Ω…“‘ΆΤ≥ωClO2ΒΡ―θΜ·–‘¥σ”ΎH2O2Θ§Dœν’ΐ»ΖΘΜ
Ι ¥πΑΗ―ΓCΓΘ

ΓΨΧβΡΩΓΩΈΣ»ΖΕ®Na2CO3ΚΆNaHCO3ΜλΚœΈο―υΤΖΒΡΉι≥…Θ§≥Τ»ΓΥΡΖίΗΟ―υΤΖ»ή”ΎΥ°ΚσΖ÷±π÷πΒΈΦ”»κœύΆ§≈®Ε»―ΈΥα30.0 mLΘ§≥δΖ÷Ζ¥”ΠΘ§≤ζ…ζCO2ΒΡΧεΜΐ(“―’έΥψ≥…±ξΉΦΉ¥Ωωœ¬ΒΡΧεΜΐΘ§≤ΜΩΦ¬«CO2‘ΎΥ°÷–ΒΡ»ήΫβ)»γœ¬±μΘΚ
Β―ι–ρΚ≈ | Δώ | Δρ | Δσ | Δτ |
―ΈΥαΧεΜΐ(mL) | 30.0 | 30.0 | 30.0 | 30.0 |
―υΤΖ÷ ΝΩ(g) | 2.96 | 3.70 | 5.18 | 6.66 |
CO2ΧεΜΐ(mL) | 672 | 840 | 896 | 672 |
(1)―υΤΖ÷–ΒΡΈο÷ ΒΡΝΩ÷°±»n(Na2CO3)ΓΟn(NaHCO3)ΘΫ________ΓΘ
(2)―ΈΥαΒΡΈο÷ ΒΡΝΩ≈®Ε»c(HCl)ΘΫ________ΓΘ