��Ŀ����
����β���dz��е���Ҫ������Ⱦ��о���������β����Ϊ������������Ҫ����
��1��������ȼ������ʱ������Ӧ��N2(g) + O2(g)  2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol��
�� 5 min�ڸ÷�Ӧ��ƽ��������(NO) = ����T ��ʱ���÷�Ӧ��ƽ�ⳣ��K = ��
�� ��Ӧ��ʼ���ﵽƽ��Ĺ����У����������и�����仯���� ������ţ���
a. ���������ܶ� b. ��������ѹǿ
c. ����Ӧ���� d. ��λʱ���ڣ�N2��NO��������֮��
��2��H2��CO���Դ���ԭNO�Դﵽ������Ⱦ��Ŀ�ġ�
��֪��N2(g) + O2(g) = 2NO(g) ?H =" +180.5" kJ��mol��1
2H2(g) + O2(g) = 2H2O(l) ?H = ��571.6 kJ��mol��1
��H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ�� ��
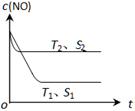
��3��������һ��ʱ�������������ı���������ѧ��Ӧ���ʡ���ͼ��ʾ��������������ʱ����Ӧ��2NO(g) + 2CO(g) 2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
�� �÷�Ӧ��?H 0 ���������������
�� �������ı����S1��S2 ������ͼ�л���c(NO) ��T1�� S2 �����´ﵽƽ������еı仯���ߡ�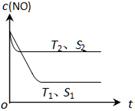
��1���� 0.2 mol��L��1��min��1 1.25 ��3�֣�
�� c d
��2��2H2(g) + 2NO(g) = N2(g) + 2H2O(l) ?H = ��752.1 kJ��mol��1��3�֣�
��3���� ��
��
���������������1������(NO)="��c(NO)��t=��n(NO)" ��V��t="5min��5L��5min=" 0.2 mol��L��1��min��1��
��������ʽ�����Ӧ������������ʵ����ı仯����ƽ��ʱ��Ӧ������������ʵ�����
N2��g��+O2��g�� 2NO��g����
2NO��g����
��ʼ��mol����6.5 mol 7.5 molO2 0
��Ӧ��mol����2.5mol 2.5mol 5mol
ƽ�⣨mol����4mol 5mol 5mol
ƽ�ⳣ��K =[c(NO)]2��c(N2)��c(O2)="(" 5mol/5L)2��(5mol/5L) ��( 4mol/5L)= 1.25��
��a�������غ㡢����������䣬���ܶ�һֱ���䣬��a����
b���÷�Ӧ�Ƿ�Ӧ�����������ķ�Ӧ���������۷�Ӧ�Ƿ�ﵽƽ��״̬����ϵ��ѹǿʼ�ղ��䣬��b����ѡcd��
��2���ɵڶ���ʽ�Ӽ�ȥ��һ��ʽ�ӣ��ó�H2(g)��NO(g)��Ӧ����N2(g)��H2O(l)���Ȼ�ѧ����ʽ����2H2(g) + 2NO(g) = N2(g) + 2H2O(l) ?H = ��752.1 kJ��mol��1��
��3������ͼ��֪T2>T1�������¶�c(NO)��������Ӧ�����ȷ�Ӧ���ʡ�H<0��
���㣺��ѧƽ���Ӱ������ �ø�˹���ɽ����йط�Ӧ�ȵļ��� ��ѧƽ�ⳣ���ĺ��� �û�ѧƽ�ⳣ�����м���
���������⿼���˻�ѧƽ����йؼ��㡢��Ӧ�ȵļ����֪ʶ�㣬��������ʽ�����л�ѧƽ����йؼ��㡢�������ʵ����������Ĺ�ϵʽ����ų����������ɣ�ע�⻯ѧƽ�ⳣ�����¶��йأ��뻯ѧ��Ӧ����ʽ����д�йأ�Ϊ�״��㡣

 ��У����ϵ�д�
��У����ϵ�д���¯������ұ��������Ҫ��������������Ҫ��ӦΪ��
Fe2O3��s��+3CO��g��⇌2Fe��s��+3CO2��g�� ��H
��1����֪����Fe2O3��s��+3C��ʯī��=2Fe��s��+3CO��g�� ��H1=+489.0 KJ��mol-1
��C��ʯī��+CO2��g��=2CO��g�� ��H2=+172.5KJ��mol-1
���H= KJ•mol-1��
��2����¯������Ӧ��ƽ�ⳣ������ʽK= ���¶����ߺ�Kֵ ������������䡱��С������
��3����T��ʱ���÷�Ӧ��ƽ�ⳣ��K=64����2L�����ܱ����������У��ֱ��±���ʾ�������ʣ���Ӧ����һ��ʱ���ﵽƽ�⡣
|
|
Fe2O3 |
CO |
Fe |
CO2 |
|
��/mol |
1.0 |
1.0 |
1.0 |
1.0 |
|
��/mol |
1.0 |
2.0 |
1.0 |
1.0 |
�ټ�������CO��ƽ��ת����Ϊ ��
������˵����ȷ���� ������ĸ����
A���������������ܶȺ㶨��������Ӧ�ﵽƽ��״̬
B����������CO��ƽ��ʱ������С����������COƽ��ʱ������
C���ס��������У�CO��ƽ��Ũ��֮��Ϊ3��2
D������Fe2O3�����������CO��ת����
��4������β���dz��п�����Ⱦ��һ����Ҫ���أ�һ��CO�����ǵĴ������ɲⶨ����β���Ƿ�����ŷű����÷����ǵĹ���ԭ������ȼ�ϵ�أ����е�����������ƣ�Y2O3��������ﯣ�ZrO2�����壬�ڸ�������״̬���ܴ���O2��������������������������ķ�ӦʽΪ ��
 ��2010?������һģ��һ����̼����������͵�����������Ҫ�Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��2010?������һģ��һ����̼����������͵�����������Ҫ�Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ�� 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol��
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���6.5 molN2��7.5 molO2����5 minʱ��Ӧ�ﵽƽ��״̬����ʱ������NO�����ʵ�����5 mol�� 2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�
2CO2(g) + N2(g) ��NO��Ũ��[c(NO)]���¶ȣ�T���������������S����ʱ�䣨t���ı仯���ߡ�