��Ŀ����
 ��2010?������һģ��һ����̼����������͵�����������Ҫ�Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ��
��2010?������һģ��һ����̼����������͵�����������Ҫ�Ļ���ԭ�ϣ���Ҳ�Ǵ�������Ҫ��Ⱦ��ۺ���������Ⱦ�ǻ�����ѧ��ǰ����Ҫ�о�����֮һ����1�����������У�SO2������SO3��2SO2��g��+O2��g���T2SO3��g������Ӧ���̵������仯��ͼ��ʾ��
��ͼ��A��ʾ
��Ӧ��������
��Ӧ��������
���ڸ÷�Ӧͨ����V2O5����������V2O5��ͼ��E�Ĵ�С�к�Ӱ�죿
����
����
������������͡�����û�С���������һ�������£���0.100molSO2��g����0.060molO2��g�������ݻ�Ϊ2L���ܱ������У���Ӧ�ﵽƽ��ʱ�����c��SO3��=0.04mol/L����������·�Ӧ��ƽ�ⳣ��K=
1.6��103
1.6��103
����2��һ�������£�������һ����̼��һ�����������ɽྻ��Դ�״���CH3OH������
��n��H2����n��CO��=
2��1
2��1
���ڼ״���Чȼ�ϵ������CH3OHΪȼ�ϣ�O2Ϊ��������KOHΪ�������ɣ��õ�طŵ�ʱ��������ӦʽΪ��
CH3OH-6e-+8OH-=CO32-+6H2O
CH3OH-6e-+8OH-=CO32-+6H2O
����3������β���dz��п�������ȾԴ֮һ�������ķ���֮һ������������������װһ������ת���������ò����ٺϽ����������������ص���ʹCO��NO��Ӧ�����ɿɲ��������̬ѭ���������壮
��д��һ����̼��һ��������Ӧ�Ļ�ѧ����ʽ��
2CO+2NO�T2CO2+N2
2CO+2NO�T2CO2+N2
������CH4����ԭNOxҲ�������������������Ⱦ�����磺
CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����=-574kJ/mol
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����=-1160kJ/mol
���ñ�״����6.72LCH4��ԭNO2��N2������������ת�Ƶ�������Ϊ
2.4NA
2.4NA
�������ӵ�������ֵ��NA��ʾ�����ų�������Ϊ260.1
260.1
kJ����������1��������ͼʾ�����仯����AΪ��Ӧ�
�ڴ����ܽ��ͷ�Ӧ�Ļ�ܣ�
�����ݻ�ѧƽ�������ʽ��ʽ����ƽ��Ũ�ȣ����ƽ�ⳣ���ĸ������õ���
��2��������ԭ���غ�����жϣ�
��ȼ�ϵ�أ�ȼ����ԭ��صĸ�����Ӧ���ǵ��ӷ���������Ӧ�������������ϵõ����ӷ�����ԭ��Ӧ��
��3���ٸ��ݷ�Ӧ���Ӧ������������д����ѧ����ʽ��
�������Ȼ�ѧ����ʽ��˹���ɵļ���Ӧ�ã�
�ڴ����ܽ��ͷ�Ӧ�Ļ�ܣ�
�����ݻ�ѧƽ�������ʽ��ʽ����ƽ��Ũ�ȣ����ƽ�ⳣ���ĸ������õ���
��2��������ԭ���غ�����жϣ�
��ȼ�ϵ�أ�ȼ����ԭ��صĸ�����Ӧ���ǵ��ӷ���������Ӧ�������������ϵõ����ӷ�����ԭ��Ӧ��
��3���ٸ��ݷ�Ӧ���Ӧ������������д����ѧ����ʽ��
�������Ȼ�ѧ����ʽ��˹���ɵļ���Ӧ�ã�
����⣺��1��������ͼʾ�����仯������AΪ��Ӧ������������SO2��O2�������������ʴ�Ϊ����Ӧ����������
�ڷ�Ӧͨ����V2O5����������V2O5��ͼ�л��E���ͣ��ӿ췴Ӧ���ʣ��ʴ�Ϊ�����ͣ�
����һ�������£���0.100molSO2��g����0.060molO2��g�������ݻ�Ϊ2L���ܱ������У���Ӧ�ﵽƽ��ʱ�����c��SO3��=0.04mol/L�����ݻ�ѧƽ�������ʽ��ʽ����ƽ��Ũ�ȣ�
2SO2��g��+O2��g���T2SO3��g��
��ʼ����mol/L�� 0.0500 0.0300 0
�仯����mol/L�� 0.04 0.02 0.04
ƽ������mol/L�� 0.01 0.01 0.04
��������·�Ӧ��ƽ�ⳣ��K=
=
=1.6��103���ʴ�Ϊ��1.6��103��
��2����һ�������£�������һ����̼��һ�����������ɽྻ��Դ�״���CH3OH��������ԭ���غ�õ���ѧ����ʽΪ��2H2+CO=CH3OH����n��H2����n��CO��=2��1��
�ʴ�Ϊ��2��1��
�ڼ״���Чȼ�ϵ������CH3OHΪȼ�ϣ�O2Ϊ��������KOHΪ�������ɣ��õ�طŵ�ʱ�������ڸ���ʧ���ӷ���������Ӧ��������ӦʽΪ��CH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
��3����һ����̼��һ��������Ӧ���ɿɲ��������̬ѭ���������壬�ж�ΪN2�����ɲ���ΪCO2��N2����Ӧ�Ļ�ѧ����ʽΪ��2CO+2NO=N2+2CO2��
�ʴ�Ϊ��2CO+2NO�T2CO2+N2��
��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����=-574kJ/mol ��
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����=-1160kJ/mol ��
���ݸ�˹���ɢ�+�ڵõ�2CH4��g��+4NO2��g���T2N2��g��+2CO2��g��+4H2O��g����=-1734kJ/mol��
�õ��Ȼ�ѧ����ʽΪ��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����=-867kJ/mol��
���ñ�״����6.72LCH4��ԭNO2��N2��n��CH4��=0.3mol��1mol������ȫ��Ӧת�Ƶ���8mol������0.3mol���鷴Ӧ����������ת�Ƶ�������Ϊ2.4NA��
�����Ȼ�ѧ����ʽ����õ�����Ϊ0.3mol��867KJ��mol=260.1KJ��
�ʴ�Ϊ��2.4NA��260.1��
�ڷ�Ӧͨ����V2O5����������V2O5��ͼ�л��E���ͣ��ӿ췴Ӧ���ʣ��ʴ�Ϊ�����ͣ�
����һ�������£���0.100molSO2��g����0.060molO2��g�������ݻ�Ϊ2L���ܱ������У���Ӧ�ﵽƽ��ʱ�����c��SO3��=0.04mol/L�����ݻ�ѧƽ�������ʽ��ʽ����ƽ��Ũ�ȣ�
2SO2��g��+O2��g���T2SO3��g��
��ʼ����mol/L�� 0.0500 0.0300 0
�仯����mol/L�� 0.04 0.02 0.04
ƽ������mol/L�� 0.01 0.01 0.04
��������·�Ӧ��ƽ�ⳣ��K=
| c2(SO3) |
| c2(SO2)��c(o2) |
| (0.04)2 |
| (0.01)2��0.01 |
��2����һ�������£�������һ����̼��һ�����������ɽྻ��Դ�״���CH3OH��������ԭ���غ�õ���ѧ����ʽΪ��2H2+CO=CH3OH����n��H2����n��CO��=2��1��
�ʴ�Ϊ��2��1��
�ڼ״���Чȼ�ϵ������CH3OHΪȼ�ϣ�O2Ϊ��������KOHΪ�������ɣ��õ�طŵ�ʱ�������ڸ���ʧ���ӷ���������Ӧ��������ӦʽΪ��CH3OH-6e-+8OH-=CO32-+6H2O��
�ʴ�Ϊ��CH3OH-6e-+8OH-=CO32-+6H2O��
��3����һ����̼��һ��������Ӧ���ɿɲ��������̬ѭ���������壬�ж�ΪN2�����ɲ���ΪCO2��N2����Ӧ�Ļ�ѧ����ʽΪ��2CO+2NO=N2+2CO2��
�ʴ�Ϊ��2CO+2NO�T2CO2+N2��
��CH4��g��+4NO2��g���T4NO��g��+CO2��g��+2H2O��g����=-574kJ/mol ��
CH4��g��+4NO��g���T2N2��g��+CO2��g��+2H2O��g����=-1160kJ/mol ��
���ݸ�˹���ɢ�+�ڵõ�2CH4��g��+4NO2��g���T2N2��g��+2CO2��g��+4H2O��g����=-1734kJ/mol��
�õ��Ȼ�ѧ����ʽΪ��CH4��g��+2NO2��g���TN2��g��+CO2��g��+2H2O��g����=-867kJ/mol��
���ñ�״����6.72LCH4��ԭNO2��N2��n��CH4��=0.3mol��1mol������ȫ��Ӧת�Ƶ���8mol������0.3mol���鷴Ӧ����������ת�Ƶ�������Ϊ2.4NA��
�����Ȼ�ѧ����ʽ����õ�����Ϊ0.3mol��867KJ��mol=260.1KJ��
�ʴ�Ϊ��2.4NA��260.1��
���������⿼���˻�ѧ��Ӧ�������仯Ӱ�����ط����жϣ�ƽ�ⳣ���ļ���Ӧ�ã�ԭ��صĵ缫�жϣ��缫��Ӧ��д����˹���ɵļ���Ӧ�ã��Ȼ�ѧ����ʽ�ļ���Ӧ�ã���Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
�����Ŀ
 ��2010?������һģ��������ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�����и����в���ȷ���ǣ���������
��2010?������һģ��������ͼ��ʾ��һЩ���ʻ�����Ĵ�����ϵ�����и����в���ȷ���ǣ���������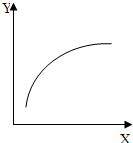 ��2010?������һģ����һ�ܱ�����ͨ��N2��H2����һ�������·�����Ӧ��N2+3H2�T2NH3����0���ﵽƽ���ֻ�ı�����һ��������X������ ����Y���ı仯һ������ͼ�����ߵ��ǣ���������
��2010?������һģ����һ�ܱ�����ͨ��N2��H2����һ�������·�����Ӧ��N2+3H2�T2NH3����0���ﵽƽ���ֻ�ı�����һ��������X������ ����Y���ı仯һ������ͼ�����ߵ��ǣ���������