��Ŀ����
����Ŀ�����������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ����Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬��B��C���γ����������η��ӣ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ���ش��������⣺
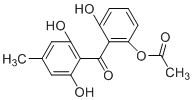
��1��������Ԫ���е縺������Ԫ�أ����̬ԭ�ӵļ۵����Ų�ͼΪ___________________����һ��������С��Ԫ����__________(��Ԫ�ط���)��
��2��C���������ǰ����Ԫ�طֱ���A�γɵĻ�����е��ɸߵ��͵�˳����___________(�ѧʽ)��
��3��BԪ�ؿ��γɶ��ֵ��ʣ�һ�־���ṹ��ͼһ��ʾ����ԭ�ӵ��ӻ�����Ϊ__________����һ�ֵľ�����ͼ����ʾ���þ����Ŀռ�������Ϊ__________��(![]() =1.732)
=1.732)

��4��DԪ���γɵĵ��ʣ��侧��Ķѻ�ģ��Ϊ__________��D�Ĵ����ξ���ֲ��ṹ��ͼ�����þ����к��еĻ�ѧ����__________ (��ѡ�����)��
�ټ��Լ� �ڷǼ��Լ� ����λ�� �ܽ�����
��5����D����������Һ�еμӹ�����ˮ���۲쵽��������______________________________��
��д���������̵����ӷ���ʽ��___________________________,__________________________��
���𰸡�![]()
![]() Cu HF��HI��HBr��HCl sp2 34% �����������ܶѻ� �٢ڢ� �����γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ Cu2++2NH3��H2O===Cu(OH)2��+2NH4+ Cu(OH)2+4NH3===[Cu(NH3)4]2++2OH
Cu HF��HI��HBr��HCl sp2 34% �����������ܶѻ� �٢ڢ� �����γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ Cu2++2NH3��H2O===Cu(OH)2��+2NH4+ Cu(OH)2+4NH3===[Cu(NH3)4]2++2OH
��������
ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ������AΪ��Ԫ�أ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����������6�����ӣ���BΪ̼Ԫ�أ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����DΪͭԪ�أ����ԭ��������֪��Cֻ�ܴ��ڵ������ڣ�B��C���γ����������ͷ��ӣ���BΪ��Ԫ�أ�
��1������Ԫ���е縺��������Cl�����̬ԭ�ӵļ۵����Ų�Ϊ3s23p5��
��2��HF���Ӽ����������е���ߣ�����±����������Է���������������е����ߣ�
��3��ͼһΪƽ��ṹ�������״�ṹ��̼̼������Ϊ120����ÿ��̼ԭ�Ӷ������3��̼ԭ�ӣ�̼ԭ�Ӳ�ȡsp2�ӻ���
���ݾ�̯�����㾧����Cԭ����Ŀ����̼ԭ��ֱ��Ϊa�����㾧����Cԭ���������̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ�����̼ԭ�ӵ��������Ϊ![]() ������������ĸ�Ϊ��a+
������������ĸ�Ϊ��a+![]() ����������������ⳤΪx����б��ĸ�Ϊ
����������������ⳤΪx����б��ĸ�Ϊ
![]() x���������ĵ��ߵľ���Ϊ
x���������ĵ��ߵľ���Ϊ![]() x��
x��![]() ���ٸ��ݹ��ɶ�������x��a�Ĺ�ϵ�������ⳤ=2x��
���ٸ��ݹ��ɶ�������x��a�Ĺ�ϵ�������ⳤ=2x��![]() =
=![]() x���ټ��㾧������������ռ�������=
x���ټ��㾧������������ռ�������=![]() ��100%��
��100%��
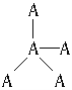
��4������CuΪ�����������ܶѻ���������λ��Ϊ12�����ͼ������ͭ����ľֲ��ṹ��ȷ���侧���к��м��Լ����Ǽ��Լ�����λ����
��5����D����������Һ�еμӹ�����ˮ���۲쵽�������������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ��
ԭ�������������������Ԫ��A��B��C��D�ֱ��ڵ�һ���������ڣ���Ȼ���д��ڶ���A�Ļ������AΪ��Ԫ�أ�Bԭ�Ӻ��������6�ֲ�ͬ���˶�״̬����������6�����ӣ���BΪ̼Ԫ�أ�D�Ļ�̬ԭ�ӵ������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӣ�Dԭ����Χ�����Ų�Ϊ3d104s1����DΪͭԪ�أ����ԭ��������֪��Cֻ�ܴ��ڵ������ڣ�B��C���γ����������ͷ��ӣ���BΪ��Ԫ�أ�
��1������Ԫ���е縺��������Cl�����̬ԭ�ӵļ۵����Ų�Ϊ3s23p5�����̬ԭ�ӵļ۵����Ų�ͼΪ![]()
![]() ������Ԫ����ֻ��CuΪ����Ԫ��ʧ����������ǿ������Ϊ�ǽ���Ԫ�أ��ʵ�һ��������С��Ԫ����Cu��
������Ԫ����ֻ��CuΪ����Ԫ��ʧ����������ǿ������Ϊ�ǽ���Ԫ�أ��ʵ�һ��������С��Ԫ����Cu��
��2��HF����֮���γ������ʹ���۷е�ϸߣ�HI��HBr��HCl����֮��ֻ�з��»�������Է�������Խ���»���Խ�е�Խ�ߣ����е��ɸߵ��͵�˳����HF��HI��HBr��HCl��
��3��ͼһΪʯī��ƽ��ṹ�������״�ṹ��̼̼������Ϊ120����ÿ��̼ԭ�Ӷ������3��̼ԭ�ӣ�̼ԭ�Ӳ�ȡsp2�ӻ���
ͼ��Ϊ���ʯ�ľ�����һ�������к�̼ԭ����Ϊ8��![]() +6��
+6��![]() +4=8����̼ԭ��ֱ��Ϊa��������Cԭ�������=8��
+4=8����̼ԭ��ֱ��Ϊa��������Cԭ�������=8��![]() ����
����![]() ��3��̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ�����̼ԭ�ӵ��������Ϊ
��3��̼ԭ������Χ��4��ԭ���γ���������ṹ������̼ԭ�����������嶥��ԭ�����ڣ�����̼ԭ�ӵ��������Ϊ![]() ������������ĸ�Ϊ��a+
������������ĸ�Ϊ��a+![]() ��=
��=![]() ��������������ⳤΪx����б��ĸ�Ϊ
��������������ⳤΪx����б��ĸ�Ϊ![]() x���������ĵ��ߵľ���Ϊ
x���������ĵ��ߵľ���Ϊ![]() x��
x��![]() ���ٸ��ݹ��ɶ�������
���ٸ��ݹ��ɶ�������![]() ��2+��
��2+��![]() x��
x��![]() ��2=��
��2=��![]() x��2��������x=
x��2��������x=![]() ���ʾ����ⳤ=
���ʾ����ⳤ=![]() ��
��![]() =
=![]() ���������
�������Ϊ��![]() ��3�������ռ�������={[8��
��3�������ռ�������={[8��![]() ����
����![]() ��3]����
��3]����![]() ��3}��100%=
��3}��100%=![]() ��
��![]() 34%��
34%��
��4������CuΪ�����������ܶѻ���������λ��Ϊ12�����ͼ������ͭ����ľֲ��ṹ��ȷ���侧���к��м��Լ����Ǽ��Լ�����λ������Ϊ���٢ڢۣ�
��5����D����������Һ�еμӹ�����ˮ���۲쵽�������������γ���ɫ�����������μӰ�ˮ�������ܽ⣬�õ�����ɫ������Һ��������Ӧ�����ӷ���ʽ��Cu2++2NH3��H2O=Cu(OH)2��+2NH4+��Cu(OH)2+4NH3===[Cu(NH3)4]2++2OH��

����Ŀ�����и��������У�X����Ҫ���ʣ�Y���������ʣ�Z��Ϊ��ȥ������Ҫ������Լ������������Լ���ȷ������� (����)
A | B | C | D | |
X | FeCl2��Һ | FeCl3��Һ | Fe | Na2SO4��Һ |
Y | FeCl3 | CuCl2 | Al | Na2CO3 |
Z | Cu | Fe | NaOH��Һ | BaCl2��Һ |
A. A B. B C. C D. D
���𰸡�C
��������A��ͭ�����Ȼ�����Ӧ�����Ȼ��������Ȼ�ͭ�������������ʣ���A����B�����߾���Fe��Ӧ�������ϳ��ӵ�ԭ���ܳ��ӣ���B����C��Al��NaOH��Һ��Ӧ����Fe���ܣ����NaOH�ܽ����˿ɳ��ӣ���C��ȷ��D�����߾���BaCl2��Һ��Ӧ��Ӧ������������ӣ���D����ѡC��
�����͡���ѡ��
��������
19
����Ŀ��ij��ѧ��ȤС��������ͼװ�ý��С�����ˮ��Ӧ����ʵ�飬�������������ʣ���ش��������⣺
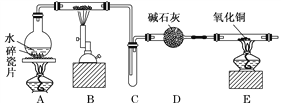
��1��Aװ�õ�������________________��B�з�Ӧ�Ļ�ѧ����ʽΪ_____________��
��2��D��������____________________________________________________________��
��3��E�������__________________________________________________________��
��4��A��B����װ����Ӧ�ȵ�ȼ_______���ľƾ��ƣ���ȼE���ƾ���֮ǰӦ���еIJ�����_______________________________________________________________________��
����Ŀ��ij�о�С�齫������SO2����ͨ��0.1mol��L-1��Ba��NO3��2��Һ�У��õ���BaSO4������Ϊ̽��������Һ�к�����������ͨ���SO2����С��ͻ�������¼���:
����һ����Һ�е�NO3-
���������Һ���ܽ��O2
��1����֤����һ
��С���漰ʵ����֤�˼���һ�������±��հ״���д���ʵ������
ʵ�鲽�� | ʵ������ | ���� |
ʵ��1����ʢ�в���O2��25ml0.1mol/LBaCl2��Һ���ձ��У�����ͨ�봿����SO2���� | ____ | ����һ���� |
ʵ��2����ʢ�в���O2��25ml0.1mol/LBa��NO3��2��Һ���ձ��У�����ͨ�봿����SO2���� | ____ |
��2��Ϊ�����о��÷�Ӧ����С�黹�����������ʵ������Һ��pH��ͨ��SO2����ı仯��������ͼ
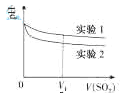
ʵ��1����ҺpH��С��ԭ����____��V1ʱ��ʵ��2����ҺpHС��ʵ��1��ԭ���ǣ������ӷ���ʽ��ʾ��_________��
��3����֤�����
�����ʵ����֤�������д��ʵ�鲽�裬Ԥ������ͽ��ۡ�
ʵ�鲽�衢Ԥ������ͽ��ۣ���Ҫ��д����������̣�____
��4�����������������Ԥ�⣺����ͬ�����£��ֱ�ͨ��������O2��KNO3��������ͬ��H2SO3��Һ����Һ����仯���Բ��ƣ�����ַ�ӳ������Һ��pHǰ��_______(����ڻ�С��)���ߣ�������________