��Ŀ����
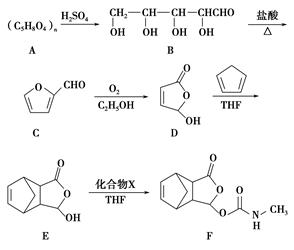
�����г��õ�ij������X�Ľṹ��ʽΪ
(1)����X�к��������ŵ�������_________________________________��
(2)����X�ɷ����ķ�Ӧ������____________________________(�����)��
a��������Ӧ����b����ԭ��Ӧ c���ӳɷ�Ӧ d����ȥ��Ӧ
(3)��֪��

����X�ĺϳ�·�����£�
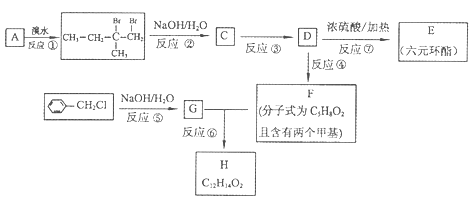
��A�Ľṹ��ʽ��______________________________________________��
�ڼ����л���C�к���̼̼˫�������õ��Լ�__________________��
a��������Һ������b�����Ը��������Һ c����ˮ d������������Һ
��D��X�Ļ�ѧ����ʽΪ________________________________________��
���л���B��ij��ͬ���칹��E�������������ʣ�
a����Ũ��ˮ��Ӧ���ɰ�ɫ��������1 mol E�������4 mol Br2��Ӧ
b�����������ʾ���л����д���̼̼˫��
��E�Ľṹ��ʽΪ_____________________________________________��
��(1)ȩ����(2)bc
(3)��CH3-CH2-CHO����ac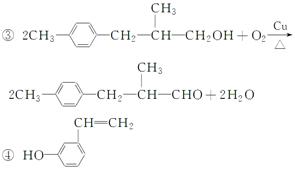
����

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 R��CHCl��CH===CH2
R��CHCl��CH===CH2 R��CH2CH2CH2CHO
R��CH2CH2CH2CHO ���ṹ���ȶ�)��������д������һ�ֵĽṹ��ʽ��______________��
���ṹ���ȶ�)��������д������һ�ֵĽṹ��ʽ��______________��
 +
+
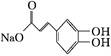 ),���Լ�X������������
),���Լ�X������������

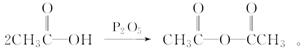 ������
������ �Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������
�Ǻϳɿ�����ҩ������Τ���м��壬����ƺ���������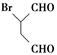 ��
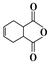
�� Ϊԭ�Ϻϳɸû�����(�úϳ�·������ͼ��ʾ����ע����Ӧ����)���ϳ�·������ͼʾ�����£�
Ϊԭ�Ϻϳɸû�����(�úϳ�·������ͼ��ʾ����ע����Ӧ����)���ϳ�·������ͼʾ�����£�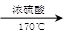 CH2===CH2
CH2===CH2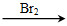
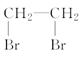
 ��������������ͼ��
��������������ͼ��
 ��-X��-YΪȡ�������Ƕ��ǻ�������������ͬ���칹�����ܷ���������Ӧ����-X�Ľṹ��ʽ������ �� ��
��-X��-YΪȡ�������Ƕ��ǻ�������������ͬ���칹�����ܷ���������Ӧ����-X�Ľṹ��ʽ������ �� ��
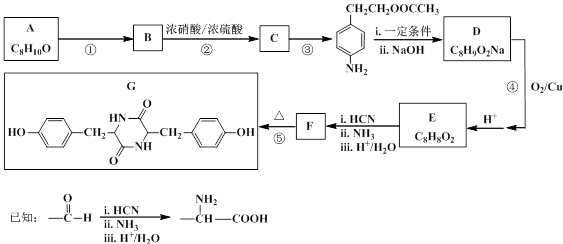
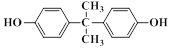 ���������
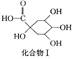
���������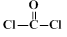 ���ۺϵõ�����д���þ�̼�����Ľṹ��ʽ______________________________��
���ۺϵõ�����д���þ�̼�����Ľṹ��ʽ______________________________��