��Ŀ����
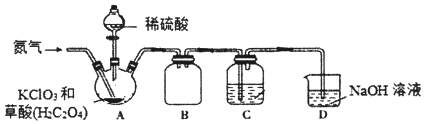
����Ŀ���������ȣ�C1O2����һ�ֻ���ɫ���壬�е�Ϊ11�棬���ȶ����ڻ�������е������������10%�Ϳ��ܷ�����ը���ڹ�ҵ�ϳ�����ˮ��������Ư����C1O2������ˮ������ˮ��Ӧ����ˮ��Һ���¶ȹ���ʱ�п��ܱ�ը��ijС�鰴������ʵ��װ���Ʊ�C1O2����֤��ijЩ���ʡ�
��ش��������⣺
��1��ʵ��ʱAװ����Ҫ�����¶�Ϊ60��80�棬��Aװ�ü��ȵķ�ʽ��___���ü��ȷ�ʽ���ŵ���_____��
��2��ͨ�백������Ҫ������������һ�ǿ����������ã�����____��
��3��Bװ�õ�������___��Cװ����������C1O2����C��Һ����ѡ������___������ţ���
����ˮ �ڱ�ˮ �۱���ʳ��ˮ
��4��װ��D��C1O2��NaOH��Һ��Ӧ�����ɵ����ʵ������������Σ�����һ��ΪNaClO2��װ��C���������������εĻ�ѧ����ʽΪ____������NaClO2��Һ���¶ȵ���38��ʱ��������NaC1O23H2O�����¶ȸ���38��ʱ��������NaClO2���벹���NaClO2��Һ���Ƶ�NaClO2����IJ������裺a��___��b��___��c��ϴ�ӣ�d�����
��5�����һ������ʵ��֤���������Ⱦ������õ�Ư���ԣ�___��
���𰸡�ˮԡ���� ���Ⱦ��ȣ����Ʒ�Ӧ�¶� ����ClO2��Ũ�ȣ����СClO2���������������ֹ��ը ��ȫƿ����ֹ���� �� 2ClO2+2NaOH=NaClO2+NaClO3+H2O �����ᾧ ���ȹ��� ȡ����Ʒ����Һ���Թ��У�ͨ��������ȣ��۲쵽Ʒ����Һ��ɫ���ټ�������ɫ����Һ����Һ�����ɫ
��������
Aװ���Ʊ�ClO2��ͨ�뵪������Ҫ������2����һ�ǿ����������ã�����ϡ�Ͷ������ȣ���ֹ��������ȵ�Ũ�ȹ��߶�������ը��Bװ��Ϊ��ȫƿ��C��������C1O2��Ϊ�����¶ȹ��ߣ�������ˮ��Dװ�ù۲쵪����ͨ�����ʣ���ֹװ�ö������ȵ�Ũ�ȹ��ߣ�Dװ������β�����Դ˽����⡣
(1)ʵ��ʱAװ����Ҫ�����¶�Ϊ60��80������Aװ�ü��ȵķ�ʽ��ˮԡ���ȣ��ü��ȷ�ʽ���ŵ������Ⱦ��ȣ����Ʒ�Ӧ�¶ȣ� �ʴ�Ϊ��ˮԡ���ȣ����Ⱦ��ȣ����Ʒ�Ӧ�¶ȣ�
(2)ͨ�뵪������Ҫ������2����һ�ǿ����������ã�����ϡ�Ͷ������ȣ���ֹ��������ȵ�Ũ�ȹ��߶�������ը���ʴ�Ϊ������ClO2��Ũ��(���СClO2���������)����ֹ��ը��
(3)Bװ��Ϊ��ȫƿ������ֹ���������ã�C��������C1O2��Ϊ�����¶ȹ��ߣ�������ˮ���ʴ�Ϊ����ȫƿ����ֹ�������ڣ�
(4)װ��D��C1O2��NaOH��Һ��Ӧ�����ɵ����ʵ������������Σ�����һ��ΪNaClO2����һ��ΪNaClO3��װ��C���������������εĻ�ѧ����ʽΪ2ClO2+2NaOH=NaClO2+NaClO3+H2O������NaClO2��Һ���¶ȵ���38��ʱ��������NaC1O23H2O�����¶ȸ���38��ʱ��������NaClO2�����NaClO2��Һ���Ƶ�NaClO2����IJ������裺a�������ᾧ�� b�����ȹ��ˣ� c��ϴ�ӣ�d������ �ʴ�Ϊ��2ClO2+2NaOH=NaClO2+NaClO3+H2O�������ᾧ�����ȹ��ˣ�
(5)�������ȿ�ʹƷ����ɫ�������Ƿ��������Ư���ԣ���ȡ����Ʒ����Һ���Թ��У�ͨ��������ȣ��۲쵽Ʒ����Һ��ɫ���ټ�������ɫ����Һ����Һ�����ɫ���ʴ�Ϊ��ȡ����Ʒ����Һ���Թ��У�ͨ��������ȣ��۲쵽Ʒ����Һ��ɫ���ټ�������ɫ����Һ����Һ�����ɫ��

����Ŀ�����ⶨijNaOH��Һ�����ʵ���Ũ�ȣ�����0.100 0 mol��L��1��HCl����Һ�ζ������(�ü�����ָʾ��)����ش��������⣺
��1���ζ�ʱ��ʢװ����NaOH��Һ����������Ϊ________��
��2���ζ����յ����ɫ�仯Ϊ____________��
��3������ѧ����ʵ���������������ƽ��ʵ�飬���ݼ�¼���£����ʱ���ı���Һ�����Ϊ__________�����������NaOH��Һ�����ʵ���Ũ��Ϊ______��(������λ��Ч����)
ʵ�� ��� | ����NaOH��Һ�����/mL | 0.100 0 mol��L��1 HCl��Һ�����/mL | |
�ζ�ǰ�̶� | �ζ���̶� | ||
1 | 25.00 | 0.00 | 26.29 |
2 | 25.00 | 1.00 | 31.00 |
3 | 25.00 | 1.00 | 27.31 |