��Ŀ����
����Ŀ��TiO2��һ�����������İ뵼������������Ч�������л���Ⱦ�������ȩ���ױ���)�ͺ���������(��NH3��CN������ת��ΪCO2��N2��С�������ʡ�
��1��Ti��̬��������Ų�ʽΪ________________��
��2����ȩHCHO���ӿռ乹��Ϊ_____��������̼ԭ�ӹ���ӻ�����Ϊ_____���м��ͦҼ��ĸ���֮��Ϊ____��
��3��������������ˮ������Ϊ����ˮ�ķ��Ӿ���_________������Ϊ___________��
��4���ױ��������ܹ���ƽ���ԭ�����Ϊ____�����������ױ�±�ؼӳɣ����Ƚ����ױ�±��ȡ�������ϵ��⣬ԭ����___________________��
��5����CN������ˮ���Լ�������NaClO�Ȱ�CN������ΪCNO����Ȼ���������������ٽ�CNO������Ϊ����Ⱦ�����塣��д����CNO����Ϊ�ȵ��������______���ӻ����ӣ�дһ�֣���
��6��Ti[(CN)4]2-��Ti2+��CN-��Cԭ���γ���λ���������ǿռ乹�ͣ�Ti[(CN)4]2-�Ľṹ�ɱ�ʾΪ_____________________��
��7��Ti��ij�������CaO��������γ������εľ����ṹ��ͼ��ʾ��Ti4+λ��������Ķ��㣬Ca2+ ��������������ģ����þ����У�Ti4+����Χ____ ��O2-����ڣ����þ������ܶ�Ϊdg/cm3���������ļ���Ϊ______pm ���ô�NA�Ĵ���ʽ��ʾ����

���𰸡� 1s22s22p63s23p63d24s2����[Ar]3d24s2�� ƽ�������� sp2 1:3 ���Է��ӣ����������ܣ� ��������ˮ���ӿ��γ����Ӽ���� 13 �����ϴ��ڴ���������˫��ƽ������ N2O��CO2��CS2��SCN����  12
12 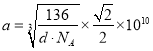
����������1��Ti��ԭ������Ϊ22��ԭ�Ӻ��������Ϊ22��λ�ڵ������ڵڢ�B�壬������d����Ti��̬��������Ų�ʽΪ1s22s22p63s23p63d24s2����[Ar]3d24s2������2����ȩHCHO���ӿռ乹��Ϊƽ�������Σ�̼�γ�3���Ҽ����µ��Ӷԣ�������̼ԭ�ӹ���ӻ�����Ϊsp2 ���м��ͦҼ��ĸ���֮��Ϊ1��3����3��������������ˮ���������Ǽ��Է��ӣ�ˮ�Ǽ����ܼ�������������ԭ����֪������������ˮ����������ˮ��Ӧ����һˮ�ϰ���ʹ�����ܽ����������ˮ����֮�����γ������ʹ�����ܽ����������4���ױ��������ܹ���ƽ���ԭ�����Ϊ7��C��������5��H������һ��H��13�����������ױ�±�ؼӳɣ����Ƚ����ױ�±��ȡ�������ϵ��⣬ԭ���DZ����ϴ��ڴ���������˫��ƽ����������5��CNO���е�����Ϊ��6+7+8+1=22����CNO����Ϊ�ȵ��������N2O��CO2��CS2��SCN���ȷ��ӻ���������6��Ti[(CN)4]2-��Ti2+��CN-��Cԭ���γ���λ���������ǿռ乹�ͣ�Ti[(CN)4]2-�Ľṹ�ɱ�ʾΪ ����7���ɾ����ṹͼ��֪������ΪCa2��������ΪO2�����þ�����ÿ������Ti4�������ĵ�O2�����ڣ�ÿ������Ϊ8���������ã�ÿ����Ϊ2���������ã������У�Ti4������Χ������O2����ĿΪ3��8/2=12���þ����У�Ti4+����Χ12 ��O2-����ڣ���ͼ������Ti4��Ϊ8��1/8=1,Ca2��Ϊ1��O2��Ϊ6��1/2=3,����߳�Ϊx����=m/v=
����7���ɾ����ṹͼ��֪������ΪCa2��������ΪO2�����þ�����ÿ������Ti4�������ĵ�O2�����ڣ�ÿ������Ϊ8���������ã�ÿ����Ϊ2���������ã������У�Ti4������Χ������O2����ĿΪ3��8/2=12���þ����У�Ti4+����Χ12 ��O2-����ڣ���ͼ������Ti4��Ϊ8��1/8=1,Ca2��Ϊ1��O2��Ϊ6��1/2=3,����߳�Ϊx����=m/v=![]() ,�����߳�Ϊ
,�����߳�Ϊ �����������ļ���Ϊ
�����������ļ���Ϊ pm��
pm��

 �ŵ������ϵ�д�
�ŵ������ϵ�д�




