ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΙΐΕ…Ϋπ τ‘ΣΥΊ―θΜ·ΈοΒΡ”Π”Ο―–ΨΩ «ΡΩ«ΑΩΤ―ß―–ΨΩΒΡ«Α―Ί÷°“ΜΘ§ ‘ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
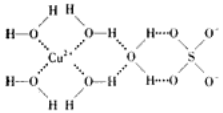
Δώ.Εΰ―θΜ·ν―ΉςΙβ¥ΏΜ·ΦΝΡήΫΪΨ” “Έέ»ΨΈοΦΉ»©ΓΔ±ΫΒ»”–ΚΠΤχΧεΩ…ΉΣΜ·ΈΣΕΰ―θΜ·ΧΦΚΆΥ°Θ§¥οΒΫΈόΚΠΜ·ΓΘ”–ΙΊΦΉ»©ΓΔ±ΫΓΔΕΰ―θΜ·ΧΦΦΑΥ°ΥΒΖ®’ΐ»ΖΒΡ «___________ΓΘ
AΘ°±Ϋ”κB3N3H6ΜΞΈΣΒ»ΒγΉ”Χε
BΘ°ΦΉ»©ΓΔ±ΫΖ÷Ή”÷–ΧΦ‘≠Ή”Ζ÷±π≤…”Οsp3ΓΔsp2‘”Μ·
CΘ°±ΫΓΔΕΰ―θΜ·ΧΦ «Ζ«ΦΪ–‘Ζ÷Ή”Θ§Υ°ΚΆΦΉ»© «ΦΪ–‘Ζ÷Ή”
DΘ°Υ°ΒΡΖ–Βψ±»ΦΉ»©ΗΏΒΟΕύΘ§ «“ρΈΣΥ°Ζ÷Ή”ΡήΡή–Έ≥…Ζ÷Ή”ΡΎ«βΦϋ
Δρ.2007Ρξ≈Β±¥ΕϊΈοάμ―ßΫ±ΈΣΖ®ΙζΩΤ―ßΦ“ΑΔΕϊ±¥ΓΛΖ―ΕϊΚΆΒ¬ΙζΩΤ―ßΦ“±ΥΒΟΓΛΗώΝ÷±¥ΗώΕϊΙ≤Ά§ΜώΒΟΘ§“‘±μ’ΟΥϊΟ«‘ΎΨό¥≈ΒγΉη–ß”ΠΘ®CMR–ß”ΠΘ©―–ΨΩΖΫΟφΒΡ≥…ΨΆΓΘΡ≥ΗΤν―–ΆΗ¥Κœ―θΜ·ΈοΘ®»γ”“ΆΦΘ©Θ§“‘A‘≠Ή”ΈΣΨßΑϊΒΡΕΞΒψΘ§AΈΜΩ…“‘ «CaΓΔSrΓΔBaΜρPbΘ§Β±BΈΜ «VΓΔCrΓΔMnΓΔFe ±Θ§’β÷÷Μ·ΚœΈοΨΏ”–CMR–ß”ΠΓΘ

Θ®1Θ©”ΟAΓΔBΓΔO±μ Ψ’βάύΧΊ βΨßΧεΒΡΜ·―ß ΫΘΚ_________ΓΘ
Θ®2Θ©“―÷ΣLaΈΣ+3ΦέΘ§Β±±ΜΗΤΒ»ΕΰΦέ‘ΣΥΊAΧφ¥ζ ±Θ§Ω…–Έ≥…Η¥ΚœΗΤν―ΩσΜ·ΚœΈοLa1-xAxMnO3, (x < 0.1)Θ§¥Υ ±“Μ≤ΩΖ÷ΟΧΉΣ±δΈΣ+4ΦέΓΘΒΦ÷¬≤ΡΝœ‘ΎΡ≥“ΜΈ¬Ε»ΗΫΫϋ”–Ζ¥Χζ¥≈-Χζ¥≈ΓΔΧζ¥≈-Υ≥¥≈ΉΣ±δΦΑΫπ τ-ΑκΒΦΧεΒΡΉΣ±δΘ§‘ρLa1-xAxMnO3÷–»ΐΦέΟΧ”κΥΡΦέΟΧΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣΘΚ_______________ΓΘΘ®”ΟΚ§xΒΡ¥ζ ΐ Ϋ±μ ΨΘ©
Θ®3Θ©œ¬Ν–”–ΙΊΥΒΖ®’ΐ»ΖΒΡ «_______________________ΓΘ
AΘ°ογΓΔΟΧΓΔ―θΖ÷±πΈΜ”Ύ÷ήΤΎ±μfΓΔdΓΔp«χ
BΘ°―θΒΡΒΎ“ΜΒγάκΡή±»ΒΣΒΡΒΎ“ΜΒγάκΡή¥σ
CΘ°ΗθΒΡΕ―ΜΐΖΫ Ϋ”κΦΊœύΆ§Θ§‘ρΤδΕ―ΜΐΖΫ Ϋ»γΆΦΘΚ
DΘ°ΟΧΒΡΒγΗΚ–‘ΈΣ1.59 Θ§CrΒΡΒγΗΚ–‘ΈΣ1.66Θ§ΥΒΟςΟΧΒΡΫπ τ–‘±»Ηθ«Ω
ΓΨ¥πΑΗΓΩ AC ABO3 Θ®1-XΘ©/X AD
ΓΨΫβΈωΓΩΔώΓΔAΘ°±Ϋ”κB3N3H6ΦέΒγΉ”Ήή ΐœύΒ»Θ§‘≠Ή” ΐ“≤œύΒ»ΜΞΈΣΒ»ΒγΉ”ΧεΘ§A’ΐ»ΖΘΜCΘ°ΦΉ»©ΓΔ±ΫΖ÷Ή”÷–ΧΦ‘≠Ή”ΨυΚ§”–3ΗωΠΡΦϋΘ§ΟΜ”–Ι¬Ε‘ΒγΉ”Θ§≤…”Οsp2‘”Μ·Θ§B¥μΈσΘΜCΘ°±ΫΓΔΕΰ―θΜ·ΧΦΖ÷Ή”ΒΡΩ’ΦδΫαΙΙΕ‘≥Τ τ”Ύ «Ζ«ΦΪ–‘Ζ÷Ή”Θ§Υ°ΚΆΦΉ»©Ζ÷Ή”ΒΡΩ’ΦδΫαΙΙ≤ΜΕ‘≥Τ τ”ΎΦΪ–‘Ζ÷Ή”Θ§C’ΐ»ΖΘΜDΘ°Υ°ΒΡΖ–Βψ±»ΦΉ»©ΗΏΒΟΕύΘ§ «“ρΈΣΥ°Ζ÷Ή”ΦδΡή–Έ≥…«βΦϋΘ§D’ΐ»ΖΘΜ¥πΑΗ―ΓACΘΜ
ΔρΓΔΘ®1Θ©”…ΆΦ1Ω…÷ΣΘ§ΨßΑϊ÷–AΈΜ”ΎΕΞΒψΘ§ΨßΑϊ÷–Κ§”–AΈΣ8ΓΝ1/8=1ΗωΘ§BΈΜ”ΎΨßΑϊΒΡΧε–ΡΘ§Κ§”–1ΗωΘ§OΈΜ”ΎΟφ–ΡΘ§ΨßΑϊ÷–Κ§”–OΒΡΗω ΐΈΣ6ΓΝ1/2=3Θ§‘ρΜ·―ß ΫΈΣABO3ΘΜΘ®2Θ©…ηLa1-xAxMnO3÷–»ΐΦέΟΧ”κΥΡΦέΟΧΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣmΚΆnΘ§‘ρ”–3(1x)+2x+3m+4nΘΫ6ΓΔm+nΘΫ1Θ§Ϋβ÷°ΒΟm=1-xΘ§n=xΘ§‘ρLa1-xAxMnO3÷–»ΐΦέΟΧ”κΥΡΦέΟΧΒΡΈο÷ ΒΡΝΩ÷°±»ΈΣΘ®1-xΘ©/xΘΜΘ®3Θ©AΘ°”…Ϋπ τ‘Ύ÷ήΤΎ±μ÷–ΒΡΈΜ÷ΟΩ…÷ΣογΓΔΟΧΓΔ―θΖ÷±πΈΜ”Ύ÷ήΤΎ±μfΓΔdΓΔp«χΘ§A’ΐ»ΖΘΜBΘ°OΓΔN‘ΣΥΊΕΦ «ΒΎΕΰ÷ήΤΎΖ«Ϋπ τ‘ΣΥΊΘ§Ά§“Μ÷ήΤΎ‘ΣΥΊΉ‘ΉσΕχ”“ΒΎ“ΜΒγάκΡή≥ ‘ω¥σ«ς ΤΘ§ΒΪN‘ΣΥΊ‘≠Ή”2pΡήΦΕ «Ακ¬ζΈ»Ε®Ή¥Χ§Θ§ΡήΝΩΫœΒΆΘ§ΒΎ“ΜΒγάκΡήΗΏ”ΎΆ§÷ήΤΎœύΝΎ‘ΣΥΊΘ§B¥μΈσΘΜCΘ°ΆΦ÷–Ε―ΜΐΖΫ ΫΈΣΟΨ–ΆΘ§C¥μΈσΘΜDΘ°‘ΣΥΊΒΡΒγΗΚ–‘‘Ϋ«ΩΘ§Ϋπ τ–‘‘Ϋ»θΘ§D’ΐ»ΖΘΜ¥πΑΗ―ΓADΓΘ

 Κ°ΦΌΧλΒΊ÷Ί«λ≥ωΑφ…γœΒΝ–¥πΑΗ
Κ°ΦΌΧλΒΊ÷Ί«λ≥ωΑφ…γœΒΝ–¥πΑΗΓΨΧβΡΩΓΩœ¬±μ «AΓΔBΓΔCΓΔDΥΡ÷÷”–ΜζΈοΒΡ”–ΙΊ–≈œΔΘΚ
A | ΔΌΡή ΙδεΒΡΥΡ¬»Μ·ΧΦ»ή“ΚΆ …ΪΘΜΔΎ±»άΐΡΘ–ΆΈΣ |
B | ΔΌ”…CΓΔHΝΫ÷÷‘ΣΥΊΉι≥…ΘΜΔΎ«ρΙςΡΘ–ΆΈΣ |
C | ΔΌ”…CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΉι≥…ΘΜΔΎΡή”κNaΖ¥”ΠΘ§ΒΪ≤ΜΡή”κNaOH»ή“ΚΖ¥”ΠΘΜΔέΡή”κDΖ¥”Π…ζ≥…œύΕ‘Ζ÷Ή”÷ ΝΩΈΣ88ΒΡθΞ |
D | ΔΌ”…CΓΔHΓΔO»ΐ÷÷‘ΣΥΊΉι≥…ΘΜΔΎ«ρΙςΡΘ–ΆΈΣ |
ΜΊ¥πœ¬Ν–Έ ΧβΘΚ
(1)A”κδεΒΡΥΡ¬»Μ·ΧΦ»ή“ΚΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ___________________ΓΘ
(2)BΨΏ”–ΒΡ–‘÷ «________(Χν–ρΚ≈)ΓΘ
ΔΌΈό…ΪΈόΈΕ“ΚΧε ΔΎ”–ΕΨ Δέ≤Μ»ή”ΎΥ° ΔήΟήΕ»±»Υ°¥σ
Δί”κΥα–‘KMnO4»ή“ΚΚΆδεΥ°Ζ¥”ΠΆ …Ϊ Δό»ΈΚΈΧθΦΰœ¬≤Μ”κ«βΤχΖ¥”Π
(3)B‘Ύ50ΓΪ60Γφ ±Θ§Ρή”κΜλΥα(≈®ΝρΥαΚΆ≈®œθΥαΒΡΜλΚœ»ή“Κ)Ζ¥”ΠΘ§ΗΟΖ¥”ΠΒΡΖ¥”Πάύ–Ά «_______________Θ§Μ·―ßΖΫ≥Χ ΫΈΣ___________________ΓΘ
(4)C”κDΖ¥”Π…ζ≥…”–œψΈΕΒΡΈο÷ ΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ _______________Θ§ΗΟΖ¥”ΠΒΡΖ¥”Πάύ–Ά «_______________ΓΘ
(5)B”κ“ΚδεΦΑΧζΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣΘΚ__________Θ§ …ζ≥…”–ΜζΈοΒΡΟϊ≥Τ «________ΓΘ
ΓΨΧβΡΩΓΩάϊ”Οœ¬ΆΦΉΑ÷Ο ’Φ·ΤχΧε≤Δ―ι÷ΛΤδΡ≥–©Μ·―ß–‘÷ Θ§’ΐ»ΖΒΡ «

―Γœν | ΤχΧε | ‘ΦΝΔΎ | œ÷œσ | Ϋα¬έ |
A | NH3 | Ζ”ΧΣ ‘“Κ | »ή“Κ±δΚλ…Ϊ | NH3ΒΡΥ°»ή“Κœ‘Φν–‘ |
B | Cl2 | Ήœ…Ϊ ·»ο ‘“Κ | »ή“ΚΝΔΦ¥Ά …Ϊ | Cl2”–―θΜ·–‘ |
C | SO2 | δεΥ° | »ή“ΚΆ …Ϊ | SO2”–Τ·ΑΉ–‘ |
D | XΘ®Ρή–Έ≥…Υα”ξΘ© | KIΒμΖέ»ή“Κ | »ή“Κ±δάΕ | X «NO2 |
ΓΨΧβΡΩΓΩ«ΑΥΡ÷ήΤΎ‘ΣΥΊAΓΔBΓΔCΓΔDΓΔEΒΡ‘≠Ή”–ρ ΐ“ά¥Έ‘ω¥σΘ§ΜυΧ§A‘≠Ή”ΚΥΆβΒγΉ”’ΦΨί3ΗωΙλΒάΘ§ΜυΧ§B‘≠Ή”ΚΥΆβΒγΉ”’ΦΨί3ΗωΡήΦΕ«“ΟΩΗωΡήΦΕ…œΒγΉ” ΐœύΒ»Θ§CΒΡΥΪ‘≠Ή”ΒΞ÷ Ζ÷Ή”÷–Π“ΦϋΚΆΠ–Φϋ ΐΡΩ÷°±»ΈΣ1:2Θ§DΒΡΉνΗΏ’ΐΜ·ΚœΦέΚΆΉνΒΆΗΚΜ·ΚœΦέ÷°ΚΆΒ»”Ύ4ΘΜΜυΧ§E‘≠Ή”ΚΥΆβ”–6ΗωΈ¥≥…Ε‘ΒγΉ”ΓΘ
Θ®1Θ©ΜυΧ§E‘≠Ή”ΒΡΚΥΆβΒγΉ”≈≈≤Φ ΫΈΣ______ΘΜΜυΧ§D‘≠Ή”ΚΥΆβΒγΉ”’ΦΨίΒΡΡήΝΩΉνΗΏΒΡΡήΦΕΖϊΚ≈ΈΣ_____________ΓΘ
Θ®2Θ©A‘ΣΥΊΒΡΗςΦΕΒγάκΡή»γœ¬ΘΚ
ΡήΦΕ(I) | I1 | I2 | I3 | I4 | I5 |
ΒγάκΡή/kJΓΛmol-1 | 800.6 | 2427 | 3660 | 25026 | 32827 |
Ζ÷Έω…œ±μ ΐΨί÷ΣΘ§œύΝΎΝΫΗωΒγΉ”ΒΡΒγάκΡή÷–Θ§I3ΚΆI4÷°Φδ≤ν“λΉν¥σΘ§Τδ÷ς“Σ‘≠“ρ «__________ΓΘ
Θ®3Θ©AΓΔBΓΔC‘ΣΥΊΒΡΉνΗΏΦέ―θΜ·ΈοΕ‘”ΠΒΡΥ°Μ·ΈοΥα–‘“ά¥Έ‘ω«ΩΘ§Τδ‘≠“ρ «________ΓΘ
Θ®4Θ©¬»‘ΣΥΊ”κAΓΔBΓΔC‘ΣΥΊΉι≥…ΒΡΙ≤ΦέΖ÷Ή”ACl3ΓΔBCl4ΓΔCCl3÷–Θ§÷––Ρ‘≠Ή”≤…”Οsp3‘”Μ·ΓΔΝΔΧεΙΙ–ΆΈΣ»ΐΫ«ΉΕ–ΈΒΡ «_______(ΧνΖ÷Ή” Ϋ)ΓΘ
Θ®5Θ©(DC)4ΈΣ»»…Ϊ–‘ΙΧΧεΘ§«“”–…ΪΈ¬–ß”ΠΓΘΒΆ”Ύ-30Γφ ±ΈΣΒ≠ΜΤ…ΪΘ§ “Έ¬œ¬ΈΣ≥»ΜΤ…ΪΘ§ΗΏ”Ύ100Γφ ±ΈΣ…νΚλ…ΪΓΘ‘ΎΒ≠ΜΤ…ΪΓζ≥»ΜΤ…ΪΓζ…νΚλ…ΪΒΡΉΣΜ·÷–Θ§ΤΤΜΒΒΡΉς”ΟΝΠ «____ΘΜ‘Ύ≥Θ―Ιœ¬Θ§(DC)4ΗΏ”Ύ130ΓφΖ÷ΫβΈΣœύ”ΠΒΡΒΞ÷ Θ§’β“Μ±δΜ·÷–ΤΤΜΒΒΡΉς”ΟΝΠ «_______ΓΘ‘ΎBΓΔCΓΔDΒΡΦρΒΞΤχΧ§«βΜ·Έο÷–Θ§ τ”ΎΖ«ΦΪ–‘Ζ÷Ή”ΒΡ «______(ΧνΖ÷Ή” ΫΘ§œ¬Ά§)Θ§≥Θ”κCu2+ΓΔZn2+ΓΔAg+Β»–Έ≥…≈δάκΉ”ΒΡ «_______________ΓΘ
Θ®6Θ©AΓΔC–Έ≥…ΝΔΖΫΨßΧεΘ§ΨßΧεΫαΙΙάύΥΤΫπΗ’ ·Θ§»γΆΦΥυ ΨΓΘ“―÷ΣΘΚΗΟΨßΧεΟήΕ»ΈΣΠ―gΓΛ©M-3Θ§NA¥ζ±μΑΔΖϋΌΛΒ¬¬ό≥Θ ΐΒΡ÷ΒΓΘ

ΔΌΗΟΨßΧεΒΡΜ·―ß ΫΈΣ__________ΓΘ
ΔΎ‘ΎΗΟΨßΧε÷–Θ§A”κC–Έ≥…Ι≤ΦέΦϋΒΡΦϋ≥Λ(d)ΈΣ_____pmΓΘ
ΓΨΧβΡΩΓΩœ¬Ν– Β―ι≤ΌΉςΚΆœ÷œσΕ‘”ΠΒΡΫα¬έ¥μΈσΒΡ «
―Γœν | Β―ι≤ΌΉςΚΆœ÷œσ | Ϋα¬έ |
A | œρ»ή“ΚX÷–ΒΈΦ”BaCl2»ή“ΚΘ§”–ΑΉ…Ϊ≥ΝΒμ…ζ≥… | »ή“ΚX÷–Ω…ΡήΚ§”–SO42- |
B | Έο÷ ΒΡΝΩ÷°±»ΈΣ2:3ΒΡœΓœθΥαΚΆœΓΝρΥα
| Ζ¥”ΠΫα χΚσΘ§ΉΕ–ΈΤΩ÷–»ή“ΚΒΡ»ή÷ «CuSO4Θ§Φ·ΤχΤΩ÷– ’Φ·ΒΫΒΡΤχΧε «NO |
C | œρ1mL≈®Ε»ΨυΈΣ0.05molΓΛL-1NaClΓΔNaIΒΡΜλΚœ»ή“Κ÷–ΒΈΦ”2ΒΈ0.01molΓΛL-1AgNO3»ή“ΚΘ§’ώΒ¥Θ§≥ΝΒμ «ΜΤ…Ϊ | Ksp(AgCl)<Ksp(AgI) |
D | “Έ¬œ¬Θ§”ΟpH ‘÷Ϋ≤βΒΟ0.1molΓΛL-1 NaHSO3»ή“ΚΒΡpH‘ΦΈΣ5 | HSO3-ΒΡΒγάκ≥ΧΕ»¥σ”ΎΤδΥ°Ϋβ≥ΧΕ» |
ΓΨΧβΡΩΓΩ“ΜΕ®Έ¬Ε»œ¬Θ§‘Ύ3ΗωΧεΜΐΨυΈΣ1.0LΒΡΚψ»ίΟή±’»ίΤς÷–Ζ¥”Π2H2 (g) +CO (g) ![]() CH3OH (g)¥οΒΫΤΫΚβΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
CH3OH (g)¥οΒΫΤΫΚβΓΘœ¬Ν–ΥΒΖ®’ΐ»ΖΒΡ «
»ίΤς | Έ¬Ε»/K | Έο÷ ΒΡΤπ Φ≈®Ε»/mol.L-l | Έο÷ ΒΡΤΫΚβ≈®Ε»Θ·mol.L-1 | ||
c(H2) | c(CO) | c(CH3OH) | c(CH3OH) | ||
I | 400 | 0.20 | 0.10 | 0 | 0.080 |
II | 400 | 0.40 | 0.20 | 0 | |
III | 500 | 0 | 0 | 0.10 | 0.025 |
A. ΗΟΖ¥”ΠΒΡΡφΖ¥”ΠΖ≈»»
B. ¥οΒΫΤΫΚβ ±Θ§»ίΤςI÷–Ζ¥”ΠΈοΉΣΜ·¬ ±»»ίΤςII÷–ΒΡ¥σ
C. ¥οΒΫΤΫΚβ ±Θ§»ίΤςII÷–c(H2)¥σ”Ύ»ίΤςIII÷–c(H2)ΒΡΝΫ±Ε
D. ¥οΒΫΤΫΚβ ±Θ§»ίΤςIII÷–ΒΡ’ΐΖ¥”ΠΥΌ¬ ±»»ίΤςI÷–ΒΡ¥σ