题目内容
(6分)实验室用50mL浓盐酸跟足量的氯酸钾固体共热制取氯气,反应的化学方程式为(未配平)KClO3+HCl——HCl+Cl2↑+H2O
(1)配平上述反应化学方程式:
KClO3+ HCl—— KCl+ Cl2↑+ H2O
(2)若产生0.1mol Cl2,则转移电子的物质的量为 mol。
(3)若反应中HCl的的利用率只有50%,当氧化产物比还原子能产物多7.1g时,求浓盐
酸的物质的量浓度。
(1)配平上述反应化学方程式:
KClO3+ HCl—— KCl+ Cl2↑+ H2O
(2)若产生0.1mol Cl2,则转移电子的物质的量为 mol。
(3)若反应中HCl的的利用率只有50%,当氧化产物比还原子能产物多7.1g时,求浓盐
酸的物质的量浓度。
1)1 6 1 3 3 (2)
(3)设生成Cl2的物质的量为 ,则
,则
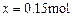
反应的HCl的物质的量为0.3mol

(3)设生成Cl2的物质的量为
 ,则
,则
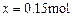
反应的HCl的物质的量为0.3mol

略

练习册系列答案
 应用题天天练四川大学出版社系列答案
应用题天天练四川大学出版社系列答案
相关题目
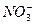 +( )H++( )e-="===( " )NO+( )H2O,该式的配平系数是( )
+( )H++( )e-="===( " )NO+( )H2O,该式的配平系数是( )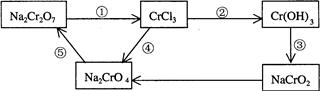
 是很强的氧化剂,在酸性溶液中它可将
是很强的氧化剂,在酸性溶液中它可将 氧化成
氧化成 。取1支试管,加入少量
。取1支试管,加入少量
 ,然后滴入2mL1 mol
,然后滴入2mL1 mol 溶液。试回答:
溶液。试回答: _______ _(填“能”或“不能”),用化学方程式回答
_______ _(填“能”或“不能”),用化学方程式回答