题目内容
用均为0.1 mol的CH3COOH和CH3COONa配制成1L混合溶液,已知其中 c (CH3COO–) > c (Na+),对该混合溶液的下列判断正确的是
| A. c (OH–) > c (H+) |
| B. c (CH3COOH) + c (CH3COO– ) = 0.2 mol/L |
| C. c (CH3COOH)> c (CH3COO– ) |
| D. c (CH3COO– )+ c (OH– ) = 0.2 mol/L |
B
混合溶液中只存在四种带电粒子:H+、CH3COO–、Na+、OH–,由电荷守恒可知:c (CH3COO–)+c (OH–) =" c" (H+) + c (Na+),由于 c (CH3COO–) > c (Na+),则 c (H+) >c (OH–),A错;
由物料守恒可知:c (CH3COOH) + c (CH3COO– ) = 0.2 mol/L,B正确;
由上述分析可知混合溶液呈酸性,也就是说CH3COOH电离的程度大于CH3COONa水解的程度,最终 c (CH3COOH) < c (CH3COO– ),C错;
由c (CH3COO–)+c (OH–) =" c" (H+) + c (Na+),其中 c (Na+)="0.1" mol/L,而c (H+)远小于0.1 mol/L,所以 c (CH3COO– ) + c (OH– ) < 0.2 mol/L,D错;
由物料守恒可知:c (CH3COOH) + c (CH3COO– ) = 0.2 mol/L,B正确;
由上述分析可知混合溶液呈酸性,也就是说CH3COOH电离的程度大于CH3COONa水解的程度,最终 c (CH3COOH) < c (CH3COO– ),C错;
由c (CH3COO–)+c (OH–) =" c" (H+) + c (Na+),其中 c (Na+)="0.1" mol/L,而c (H+)远小于0.1 mol/L,所以 c (CH3COO– ) + c (OH– ) < 0.2 mol/L,D错;

练习册系列答案
相关题目

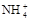 )最大的是( )
)最大的是( )