��Ŀ����
����Ŀ�����������������������γ����������꣬�������γɹ⻯ѧ�������Ժ��е�������ķ������д�����
(1)������������Һ�������շ����еĵ��������Ӧ�Ļ�ѧ����ʽ���£�
NO2��NO��2NaOH===2NaNO2��H2O��
2NO2��2NaOH===NaNO2��NaNO3��H2O��
�ڷ�Ӧ���У���������________________����ԭ����________________���ڷ�Ӧ���У��������ͻ�ԭ�������ʵ���֮��Ϊ___________________________________________��
(2)����β���к���CO��NO���������������ʶԴ�������Ⱦ�ķ����ǰ�װ��ת������ʹ���Ƿ�����Ӧ����N2��CO2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
(3)�ɷ�Ӧ���ж϶��������Ƿ�Ϊ����������(������������������)________��ԭ����________________________________________________________________________��
(4)Ŀǰ��һ��������������һ�������£��ð�������������ת��Ϊ����Ⱦ�����ʣ���д���÷�Ӧ�Ļ�ѧ����ʽ��________________________________________________���÷�Ӧ�У���������________������������________������1.4 mol����ʱ��ת�Ƶ��ӵ����ʵ���Ϊ_______________________________________________��
(5)Ϊ�˱�����������ҵ�ϳ�ͨ��NH3ʹ����������Ͱ�ת��Ϊ����N2������NO2��NO�Ļ������3 L��ͨ��3 L(ͬ��ͬѹ��)NH3��ǡ��ʹ����ȫת��ΪN2����ԭ���������NO2��NO�����ʵ���֮��Ϊ______��
���𰸡�NO2 NO 1��1 2NO��2CO![]() N2��2CO2 ���� ��Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯 6NO2��8NH3
N2��2CO2 ���� ��Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯 6NO2��8NH3![]() 7N2��12H2O NO2 N2 4.8 mol 1��1
7N2��12H2O NO2 N2 4.8 mol 1��1
��������
(1)������ԭ��Ӧ���������õ��ӻ��ϼ۽��ͣ���ԭ��ʧ���ӻ��ϼ����ߣ�
(2)���ݵ����غ��Ԫ���غ���ƽ����ʽ��
(3)�÷�Ӧ�ж����������ϼ۷����仯��
(4)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪�������Ͷ���������Ӧ�����ɵ�����Ⱦ������Ϊ������ˮ��
(5)���ݵ����غ���м��㡣
(1)�ڷ�Ӧ����NO2�еĵ�Ԫ�ػ��ϼ۽�������������NO�е�Ԫ�ػ��ϼ���������ԭ�����ڷ�Ӧ�����������ͻ�ԭ������NO2�����ǵ����ʵ���֮��Ϊ1��1���ʴ�Ϊ��NO2��NO��1��1��
(2)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪��һ��������һ����̼��Ӧ�����ɵĶԴ�������Ⱦ������ӦΪ�����Ͷ�����̼�����ݵ����غ��Ԫ���غ�ɵ÷���ʽΪ��2NO��2CO![]() N2��2CO2���ʴ�Ϊ��2NO��2CO
N2��2CO2���ʴ�Ϊ��2NO��2CO![]() N2��2CO2��
N2��2CO2��
(3)����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯������������������������ʴ�Ϊ�����ǣ���Ϊ����������Ӧ���������Σ��ҵ�Ԫ�ػ��ϼ۷����仯��
(4)����ԭ���غ㶨�ɽ����Ŀ��Ϣ��֪�������Ͷ���������Ӧ�����ɵ�����Ⱦ������Ϊ������ˮ��NO2�У�4�۵�N��NH3�У�3�۵�N��ԭΪN2��0�۵�N��ʣ�µ��⡢��Ԫ�ؽ�ϳ�ˮ�������������NO2�����������ԭ�����ΪN2������7 mol N2ʱ��ת�Ƶ���24 mol��������1.4 mol N2ʱ��ת�Ƶ���4.8 mol���ʴ�Ϊ��6NO2��8NH3![]() 7N2��12H2O��NO2��N2��4.8 mol��
7N2��12H2O��NO2��N2��4.8 mol��
(5) ��NO2�����Ϊx����NO�����Ϊ(3 L��x)��NO2![]()
![]() N2��NO
N2��NO![]()
![]() N2��NH3
N2��NH3![]()
![]() N2�����ݵ�ʧ�����غ�ԭ���ã�
N2�����ݵ�ʧ�����غ�ԭ���ã�![]() �����x��1.5 L����Ϊͬ������������ʵ���֮�ȵ��������֮�ȣ�����n(NO2)��n(NO)��1��1���ʴ�Ϊ��1:1��
�����x��1.5 L����Ϊͬ������������ʵ���֮�ȵ��������֮�ȣ�����n(NO2)��n(NO)��1��1���ʴ�Ϊ��1:1��

 �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�����Ŀ��I.�о�������ʴ�ͷ�����ԭ��������ʵ���塣
(1)��ͼΪ�˽̰�̲���̽��������������ʴ��װ�á�ij��ȤС�鰴��װ��ʵ�飬������Һ�����������������д�ʩ���Ը���������۲쵽ˮ�������������______(�����)��
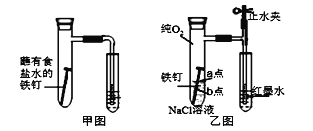
A.�ô����������Թ��ڿ��� B.�þƾ��Ƽ����Թ�����¶�
C.�������������ۺ�̿�ۻ�Ϸ�ĩ D.���ɸ�ϸ�ĵ��ܣ�ˮ�еμӺ�īˮ
(2)��С�齫��ͼװ�øĽ�����ͼװ�ò�����ʵ�飬�����к�īˮҺ���߶���ʱ��ı仯���±������������жϸ�ʴ��������ʱ����______(�����ӿ�����������������������)������ΪӰ������Ϊ_______��
ʱ��/min | 1 | 3 | 5 | 7 | 9 |
Һ���߶�/cm | 0.8 | 2.1 | 3.0 | 3.7 | 4.2 |
(3)Ϊ̽��������ʴʵ�� a��b �����������ķ�Ӧ����������ʵ�飬����ɱ���հף�
ʵ����� | ʵ������ | ʵ����� |
��NaCl��Һ�еμ�2~3�η�ָ̪ʾ�� | a�㸽����Һ���ֺ�ɫ | a��缫��ӦΪ_____ |
Ȼ���ٵμ�2~3�����軯����Һ | b����Χ������ɫ���� | b��缫��ӦΪ Fe -2e-=Fe2+ |
(4)�������װ���о������Ի����и�ʴ����Ҫ��ʽ���ⶨ��ƿ����ѹ�Ϳ��������������������ʱ��仯��ͼ����ͼ�пɷ�����t1~t2֮����Ҫ����_______��ʴ(������������)��ԭ��_______��
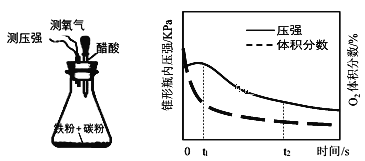
(5)���������ۻ���һ�ֵ绯ѧ������������Fe����������H2SO4��Һ�У�һ��������Fe�ۻ��γ�����Fe3O4����Ĥ����д���������缫��Ӧʽ______��
II.��֪���ᾧ��(H2C2O4��XH2O)������ˮ�����������Ը��������Һ��ȫ��Ӧ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O������������ԭ�ζ����ⶨ���ᾧ��Ľᾧˮ������X���������£�
���÷�����ƽ��ȡ���ᾧ��1.260g���������Ƴ�100.00mL���������Һ
������Һ����ȡ25.00mL���������Һ����ƿ�У����������������ữ
����Ũ��Ϊspan>0.1000mol/L��KMnO4 ����Һ���еζ������ν�����£�
��һ�εζ� | �ڶ��εζ� | �����εζ� | |
������Һ���(mL) | 25.00 | 25.00 | 25.00 |
����Һ���(mL) | 9.99 | 10.01 | 10.00 |
��֪H2C2O4����Է�������Ϊ90����ش��������⣺
(1)�ζ�ʱ��KMnO4����ҺӦ��װ��______(������ʽ��������ʽ��)�ζ����С�
(2)����ζ��յ�ı�־��________��
(3)�����������ݼ���X=_______��
(4)������(��ƫ�ߡ�ƫ�ͻ���Ӱ��)�����ζ���ʼʱ���ӵζ��̶ܿȣ��ζ�����ʱ���ӵζ��̶ܿȣ���Xֵ________��