��Ŀ����
�ݱ�������300�桢70MPa���ɶ�����̼�������ϳ��Ҵ��ѳ�Ϊ��ʵ��
2CO2(g)+6H2(g) CH3CH2OH(g)+3H2O(g)
���������������
CH3CH2OH(g)+3H2O(g)
���������������
A��ʹ��Cu-Zn-Fe�����ɴ���������Ч��
B����Ӧ����300����п��Ʋ�÷�Ӧ�����ȷ�Ӧ
C���������CO2��������H2��ת����
D����ƽ���������з����CH3CH2OH��H2O�����CO2��H2��������
���𰸡�
B
����������

��ϰ��ϵ�д�
�����Ŀ
 CH3CH2OH��g��+3H2O��g�� ��������������ǣ�������
CH3CH2OH��g��+3H2O��g�� ��������������ǣ�������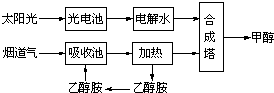

 CH3CH2OH(g)+3H2O(g)
���������������
CH3CH2OH(g)+3H2O(g)
���������������