��Ŀ����
13���������¸���˵������ȷ��������ˮ���⡢��ԭ�Ӽ���Ի�ѧ������
�ڷ����м���Խ����Խ���������Խ�ȶ�
�۸ɱ����ȱ�Ϊ����͵��������˷������Ӽ�����������ͬ������
��H2S��CS2���Ӷ��Ǻ��м��Լ��ļ��Է���
����̬˫ԭ�ӷ����п϶������ŦҼ������ܴ����Ŧм�
��CH4��NH3��CCl4���ӵĿռ乹�������������Σ�������
| A�� | �ڢۢ� | B�� | �ڢ� | C�� | �ۢ� | D�� | �٢ܢ� |
���� �ٷ��Ӽ���������
�ڷ����м���Խ����Խ��Խ�ȶ���
�۸ɱ��͵ⶼΪ���Ӿ��壻
��H2SΪ���Է��ӣ�CS2Ϊ�Ǽ��Է��ӣ�
����̬˫ԭ�ӷ����п϶������ŦҼ�����Ϊ������������Ŧм���
��NH3�Ŀռ乹���������Σ�
��� �⣺��ˮ�����ڴ��ڹ��ۼ��������Ӽ����������ʴ���
�ڷ����м���Խ����Խ�̣�����Խ�ȶ����ʴ���
�۸ɱ��͵ⶼΪ���Ӿ��壬�˷����Ӽ�������������ȷ��
��H2SΪ���Է��ӣ�CS2Ϊ�Ǽ��Է��ӣ��ʴ���
�ݵ�����������ŦҼ����м�������ȷ��
��NH3�Ŀռ乹���������Σ��ʴ���
��ѡC��
���� ���⿼�龧��Ĺ�����������������Ϊ�߿���Ƶ���㣬�����ڻ���֪ʶ�Ŀ��飬�ܺܺõ�����ѧ���Ŀ�ѧ������ע����վ���������������������Ĺ�ϵ���ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
3���Կ��淴Ӧƽ�⣺A��g��+3B��g��?2C��g����H��0����������������ǣ�������
| A�� | �����¶ȣ�v��������v���棩������v���������ĸ��� | |
| B�� | ����ѹǿ��v��������v���棩������v���������ĸ��� | |
| C�� | ����A��Ũ�ȣ�v������������v���棩���С | |
| D�� | �����ʵ�������������v��������v���棩ͬʱ����������ı�����ͬ |
4�����������м��ܸ�ϡH2SO4��Ӧ�����ܸ�����������Һ��Ӧ���ǣ�������
��NaHCO3��Al2O3��Al��OH��3 ��Al ��AlCl3��NaAlO2��
��NaHCO3��Al2O3��Al��OH��3 ��Al ��AlCl3��NaAlO2��
| A�� | �ڢۢܢ� | B�� | �ڢۢܢ� | C�� | �٢ڢۢܢ� | D�� | �٢ڢۢ� |
1�����й��ڻ�ѧ����ı�ʾ��ȷ���ǣ�������
| A�� | �������Ƶ���ʽ��Na${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$$\underset{\stackrel{••}{O}}{••}$${\;}_{•}^{•}$Na | |
| B�� | ������35��������45����ԭ�ӣ�${\;}_{35}^{80}$Br | |
| C�� | �����ӽṹʾ��ͼ��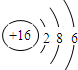 | |
| D�� | ��ϩ�Ľṹ��ʽ��C2H4 |
18�����и�������������Һ�д���������ǣ�������
| A�� | K+��H+��SO42-��OH- | B�� | Na+��H+��CO32-��Cl- | ||
| C�� | Na+��Ca2+��NO3-��CO32- | D�� | Na+��K+��Cl-��SO42- |
15������˵����ȷ���ǣ�������
| A�� | 18 gˮ���������ӵ����ʵ���Ϊ10 mol | |
| B�� | N2��CO��Ħ��������ȣ�����28 | |
| C�� | 1 mol CO2������Ϊ44 g•mol-1 | |
| D�� | ����������Ħ��������Ϊ98 g |
