��Ŀ����
��16�֣������������ҹ�����Դ������ռ�ϴ���أ������ŷų���SO2�����һϵ�л�������̬���⣬ֱ���ŷź�SO2���������γ����꣬Σ��������
��1���û�ѧ����ʽ��ʾSO2�γ�����������ķ�Ӧ�� ��
��2����ҵ����Na2SO3��Һ���������е�SO2��������ͨ��1.0 mol��L-1��Na2SO3��Һ����ҺpH���ϼ�С������ҺpHԼΪ6ʱ������SO2�����������½���Ӧ�������ռ���
�� ��ʱ��Һ��c(SO32�C)��Ũ����0.2 mol��L-1������Һ��c(HSO3�C)��_______mol?L-1��
�� ��pHԼΪ6�����ռ���ͨ��������O2���ɽ����е�NaHSO3ת��Ϊ�������ʣ���Ӧ�Ļ�ѧ����ʽ�� ��
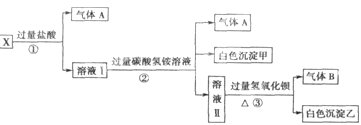
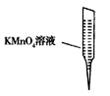
�� ij�о�С��Ϊ̽����ߺ���������SO2��
����Ч�ʵĴ�ʩ��ģ��ʵ�����պ���������ʵ������ͼ��ʾ���� ��
���������SO2������Ч�ʡ�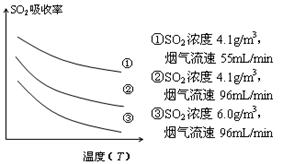
��3�������ֿ��ŵ�Na2SO3ҩƷ�Ѳ��ֱ�����
�������û�ѧС��������֪Ũ�ȵ�����KMnO4
��Һ��ȷ���京�������岽�����£�
����i����ȡ��Ʒ1.000 g��
����ii������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
����iii����ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У���0.01000 mol��L��1 KMnO4����Һ�ζ����յ㡣
�����������������ظ�2�Ρ�
�� д������iii��������Ӧ�����ӷ���ʽ___________________________________��
�� ������0.01000 mol��L��1 KMnO4��Һʱ�����Ӷ��ݣ������ղ��ҩƷ��Na2SO3�ĺ���________(�ƫ����ƫС������Ӱ�족)��
�� ijͬѧ����������������еζ�ʵ��(�гֲ�����ȥ)��������������_ (����ĸ)��

A B C D E
�� �ζ�������±���ʾ��
| �ζ����� | ������Һ �����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 2.20 | 20.20 |
��16�֣�
��1��SO2��H2O H2SO3��2H2SO3��O2��2H2SO4 ��2�֣�
H2SO3��2H2SO3��O2��2H2SO4 ��2�֣�
����2SO2��O2  2SO3��SO3��H2O��H2SO4 ��2SO2��O2��2H2O��2H2SO4��
2SO3��SO3��H2O��H2SO4 ��2SO2��O2��2H2O��2H2SO4��
��2���� 1.6 ��2�֣�
�� 2NaHSO3��O2��Na2SO4��H2SO4 ��2�֣�
�� ���ͺ����������¶ȣ����С�������������٣� ��2�֣�
��3���� 2MnO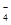 ��5SO
��5SO ��6H����2Mn2����5SO
��6H����2Mn2����5SO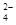 ��3H2O�� ��2�֣�
��3H2O�� ��2�֣�
�� ƫ�� ��2�֣� �� AC ��2�֣�©ѡ��1�֣���ѡ0�֣�
�� 63.00% ��63.0% ��2�֣�д��63%����1�֣�
���������������1��������������ˮ���������ᣬ������������е�������Ӧ�������ᣬ�ù��̵Ļ�ѧ����ʽΪSO2��H2O H2SO3��2H2SO3��O2��2H2SO4
H2SO3��2H2SO3��O2��2H2SO4
��2���ٸ��ݵ���غ㣬�ɵ�c(H��)+ c(Na+)= c(OH��)+2 c(SO32�C)+c(HSO3�C)������c(HSO3�C)= c(H��)+ c(Na+)- c(OH��)-2 c(SO32�C)=��1.0+10-6-10-8-2��0.2��mol��L-1������c(HSO3�C)����Ϊ1.6mol/L��
���ɢٿ�֪NaHSO3����Һ�����ԣ���������Ӧ�����ɵ�2������Ӧ�������������ᣬ��ѧ����ʽΪ2NaHSO3��O2��Na2SO4��H2SO4��
����ͼ��֪��������������������¶����߶����ͣ������ߵĶ����������������ߣ�������������С���������SO2������Ч�ʿ��Խ��ͺ����������¶ȣ����С�������������٣���
��3���ٸ��������Һ���������Ʒ���������ԭ��Ӧ���������ơ������̡�ˮ�����Բ���iii��������Ӧ�����ӷ���ʽ2MnO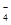 ��5SO
��5SO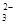 ��6H����2Mn2����5SO
��6H����2Mn2����5SO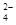 ��3H2O��
��3H2O��
�����Ƹ��������Һʱ���Ӷ��ݣ���������Һ��Ũ��ƫ�ͣ��ζ�ʱ���ĸ��������Һ�������ƫ��������ղ��ҩƷ��Na2SO3�ĺ���ƫ��
�۸���ʵ�鲽���֪����Ʒ��Һ������ƿ�У��ζ���Һ���ڵζ����ڣ�������ؾ���ǿ�����ԣ�����Ӧʹ����ʽ�ζ��ܣ����������������AC��
���ɱ������ݿ�֪3��ʹ�ø��������Һ������ֱ���20.01mL��19.99mL��18.00mL��������������ǰ������ϴ���ȥ����ǰ�����ƽ��ֵ���㣬ƽ��ֵ��20.00mL������2MnO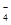 ��5SO
��5SO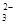 ������Ʒ���������Ƶ�����Ϊ0.02L��0.01000 mol��L��1��2.5��10��126g/mol=0.63g��������Ʒ���������Ƶ�����������0.63g/1.0000g��100%=63.00%��
������Ʒ���������Ƶ�����Ϊ0.02L��0.01000 mol��L��1��2.5��10��126g/mol=0.63g��������Ʒ���������Ƶ�����������0.63g/1.0000g��100%=63.00%��
���㣺���黯ѧ����ʽ����д����Һ����Ե��жϣ�����Ũ�ȵļ��㣬�ζ��������жϼ�����

��֪��ӦKClO3+6HCl = KCl+3Cl2��+3 H2O�������ж���ȷ����
| A��KClO3������ |
| B���������뻹ԭ��֮��Ϊ1��6 |
| C������������Cl2 |
| D��HCl�ڻ�ѧ��Ӧ��ֻ������ԭ�� |
���з�Ӧ������������ԭ��Ӧ���������ȷ�Ӧ����
| A������ϡ����ķ�Ӧ | B�������������е�ȼ�շ�Ӧ |
| C�����ȵ�ľ̿��CO2��Ӧ | D��Ba(OH)2��8H2O������NH4Cl����ķ�Ӧ |
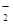 ��MnO
��MnO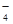 ��CO
��CO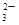 ��SO
��SO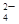 �е�������������ɣ�ȡ��Һ������ͼʵ�飺
�е�������������ɣ�ȡ��Һ������ͼʵ�飺