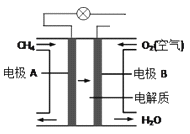
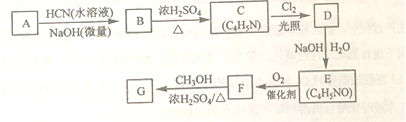
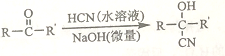
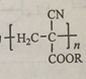��Ŀ����
����Ŀ����1������6.02��1023��H��H2O�������ʵ�����________ mol������0.4 molAl3����Al2(SO4)3��Һ������SO42���ĸ�����________
��2��0.3 mol NH3����������ԭ������_______ molH2O����������ԭ������ȡ�
��3��ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ__________��
��4��ij�ᾧˮ����Ļ�ѧʽΪA��nH2O��A����Է�������ΪM���罫a g�û�����������ᾧˮȫ��ʧȥ��ʣ��IJ���Ϊb g����n��________��
��5����ͬ��ͬѹ�£�ij����X��H2������ܶ�֮��Ϊ14��1��������X�ķ���ʽ������_______________������д�����֣���
���𰸡� 0.5 3.612��1023 0.4 4��3 (a-b)M/18b CO��N2
��������(1)6.02��1023��H�����ʵ���n=![]() =
=![]() =1mol������1molˮ�к�2mol��ԭ�ӣ��ʺ�1mol��ԭ�ӵ�ˮ�����ʵ���Ϊ0.5mol������0.4 molAl3������Һ��n[Al2(SO4)3]=
=1mol������1molˮ�к�2mol��ԭ�ӣ��ʺ�1mol��ԭ�ӵ�ˮ�����ʵ���Ϊ0.5mol������0.4 molAl3������Һ��n[Al2(SO4)3]= ![]() n(Al3��)=
n(Al3��)= ![]() ��0.4 mol=0.2mol������SO42���ĵ����ʵ���Ϊ0.2mol��3=0.6mol����ĿΪ0.6mol��6.02��1023mol1= 3.612��1023���ʴ�Ϊ��0.5mol��3.612��1023��
��0.4 mol=0.2mol������SO42���ĵ����ʵ���Ϊ0.2mol��3=0.6mol����ĿΪ0.6mol��6.02��1023mol1= 3.612��1023���ʴ�Ϊ��0.5mol��3.612��1023��
(2)0.3mol NH3�����к��е�ԭ�ӵ����ʵ���Ϊ0.3mol��4=1.2mol����n(H2O)= ![]() =0.4mol���ʴ�Ϊ��0.4��
=0.4mol���ʴ�Ϊ��0.4��
(3)NH3��CH4����ԭ�Ӹ���֮��Ϊ3��4����ҪʹNH3��CH4����ͬ��Ŀ��Hԭ�ӣ���NH3��CH4�����ʵ���֮��Ϊ4��3���ʴ�Ϊ��4��3��
(4)����ʱ���ᾧˮ����AnH2O�ֽ⣬ag�û�����������ᾧˮȫ��ʧȥ��ʣ��IJ���Ϊbg����m(A)=bg��m(H2O)=ag-bg����n(A)= ![]() mol��n(H2O)=
mol��n(H2O)= ![]() mol����n=
mol����n=![]() =
=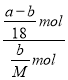 =
=![]() ���ʴ�Ϊ��
���ʴ�Ϊ�� ![]() ��
��
(5)��ͬ��ͬѹ�£�ij����X��H2������ܶ�֮��Ϊ14��1������ͬ��ͬѹ�£�������ܶ�֮�ȵ�����Է�������֮�ȣ����������Է�������=14��2=28�������������CO��N2��C2H4�ȣ��ʴ�Ϊ��CO��N2(���������𰸾���)��

 �ʰ�Ӣ��ͬ����ϰ��ϵ�д�
�ʰ�Ӣ��ͬ����ϰ��ϵ�д� ѧϰʵ����ϵ�д�
ѧϰʵ����ϵ�д�