��Ŀ����
����Ŀ��Ԫ�����ڱ��е�VIIA��Ԫ�صĵ��ʼ��仯�������;�㷺��
��1������Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж�������____(�����)��
a��Cl2��Br2��I2���۵� b��Cl2��Br2��I2��������
c��HCl��HBr��HI�����ȶ��� d��HCl��HBr��HI������
��2����Ԫ�ؿ�Ԥ����״���״�����ҹ��г��ϵ�ʳ�ξ����˵⣨KIO3������ͬѧ����ʳ���Ƿ�ӵ���������£�
![]()
������ƷΪ�ӵ�ʳ�Σ�������ӦΪ_____���˷�����KI��������_____��
����ͬѧȡ��NaCl����Ʒ������ʵ�飬Ҳ���������Ե�����ԭ����____��
�۱�ͬѧ����µļ���ӵ�ʳ�εķ������������£�
![]()
�˷�����һ���漰��Ӧ�����ӷ���ʽΪ____��
��3����֪��Ӧ2HBr(g) = H2(g) + Br2(g) ��H= +102 kJ��mol��1��
��1molH2(g)��1molBr2(g)�����л�ѧ������ʱ�ֱ���Ҫ����436kJ��200kJ����������1molHBr(g)�����л�ѧ������ʱ�����յ�����Ϊ___kJ��
��ij�¶��£������Ϊ2L���ܱ�������ͨ��amol HBr���壬10min����Br2������Ũ��Ϊbmol/L����˶�ʱ������(HBr)=____��
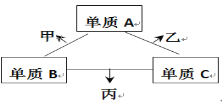
��4��һ����������ˮ��Һ��1 mol Cl����ClOx��(x��1��2��3��4)������(kJ)��Դ�С��ͼ��ʾ��

B��A��C��Ӧ���Ȼ�ѧ����ʽΪ____(�����ӷ��ű�ʾ)��
���𰸡�bc ��Һ���� ����ԭ������IO3����ԭΪI2 �����е�O2��I������Ϊ��I2 3SO32��+IO3��=3SO42��+I�� 369kJ 0.2bmol��L��1��min��1 3ClO��(aq) =2Cl��(aq) +ClO3��(aq) ��H=��117kJ��mol��1
��������
��1��a.Cl2��Br2��I2���۵�������������Է�����������������Ӽ�������������ǽ�����ǿ���أ���a����
b.ͬһ����Ԫ�أ�Ԫ�صķǽ�����Խǿ���䵥�ʵ�������Խǿ����b��ȷ��
c.ͬһ����Ԫ�أ�Ԫ�صķǽ�����Խǿ�����⻯����ȶ���Խǿ����c��ȷ��
d.ͬһ����Ԫ�أ�Ԫ�صķǽ�����Խǿ������������ˮ��������Խǿ�����⻯��ˮ��Һ�������أ���d����
�ʴ�Ϊ��bc��
��2��������ƷΪ�ӵ�ʳ�Σ��ӵ����м���KIO3��KIO3��KI�����������·�����Ӧ���ɵ��ʵ⣬��Ӧ����ʽΪ��KIO3+5KI+3H2SO4=3K2SO4+3I2+3H2O�����ɵĵ��ʵ�����������Һ��Ϊ��ɫ���÷�Ӧ��KI�е�Ļ��ϼ���-1������Ϊ0�ۣ����ϼ����ߣ�����ԭ������IO3����ԭΪI2���ʴ�Ϊ����Һ����������ԭ������IO3����ԭΪI2��
��ȡ��NaCl����Ʒ������ʵ�飬��ҺҲΪ��ɫ��ԭ���ǿ����е�O2��I������ΪI2��I2���������ۣ���ҺҲΪ��ɫ���ʴ�Ϊ�������е�O2��I������Ϊ��I2��
�۵�һ����Na2SO3��KIO3�����������·�����Ӧ��KIO3���������ԣ�Na2SO3���л�ԭ�ԣ�Na2SO3��SԪ����+4��������+6�ۣ�KIO3��IԪ����+5�۽��͵�-1�ۣ��ɻ��ϼ����������غ�(�����غ�)��ƽ����ʽ��3Na2SO3+KIO3=KI+3Na2SO4�����ӷ���ʽΪ��3SO32��+IO3��=3SO42��+I�����ʴ�Ϊ��3SO32��+IO3��=3SO42��+I����
��3����1molH2��g����ѧ������ʱ��Ҫ���յ�����Ϊ436kJ��1molBr2��g����ѧ������ʱ��Ҫ���յ�����Ϊ200kJ��1molHBr��g����ѧ������ʱ��Ҫ���յ�����ΪakJ��H2��g��+Br2��g���T2HBr��g�� ��H=-102kJ��mol-1��������H=��Ӧ����ܼ���-��������ܼ��ܣ���-102kJ/mol=436kJ/mol+200kJ/mol-2akJ/mol�����a=369���ʴ�Ϊ��369kJ��
��ij�¶��£������Ϊ2L���ܱ�������ͨ��amolHBr���壬10min����Br2������Ũ��Ϊbmol/L���������ʵĸ����֪����(Br2)=![]() =
=![]() =0.1bmol��L��1��min��1�����ݷ�Ӧ����ʽ������֮�ȵ��ڻ�ѧ������֮�ȣ�������(HBr)=2��(Br2)=0.2bmol��L��1��min��1���ʴ�Ϊ��0.2bmol��L��1��min��1��
=0.1bmol��L��1��min��1�����ݷ�Ӧ����ʽ������֮�ȵ��ڻ�ѧ������֮�ȣ�������(HBr)=2��(Br2)=0.2bmol��L��1��min��1���ʴ�Ϊ��0.2bmol��L��1��min��1��
��4��B��A+C������ת�Ƶ����غ�ø÷�Ӧ����ʽΪ3ClO-��aq��=ClO3-��aq��+2Cl-��aq������Ӧ��=��63kJ/mol+2��0kJ/mol��-3��60kJ/mol=-117kJ/mol�����Ը��Ȼ�ѧ��Ӧ����ʽΪ3ClO-��aq��=ClO3-��aq��+2Cl-��aq�� ��H=-117kJ/mol���ʴ�Ϊ��3ClO-��aq��=ClO3-��aq��+2Cl-��aq�� ��H=-117kJ/mol��