��Ŀ����
��������HCl ��NaOH ��CH3COOH ��NH3��H2O ��CH3COONa ��NH4Cl
��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01 mol/L HCl��Һ��PH= ��PH=11��CH3COONa��Һ����ˮ���������c(OH��) = ��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ�� ������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ ��
��4������PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
��5�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺
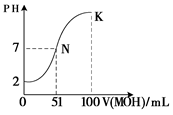
����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ������������
�� K���Ӧ����Һ�У�
c(M��)��c(MOH)= mol��L��1��
��1������������ʵ��� ����Һ�ʼ��Ե��� ������ţ���
��2��������0.01 mol/L HCl��Һ��PH= ��PH=11��CH3COONa��Һ����ˮ���������c(OH��) = ��
��3�������ӷ���ʽ��ʾCH3COONa��Һ�ʼ��Ե�ԭ�� ������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊ ��
��4������PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���m n ������ڡ����ڡ�С�ڡ�����
��5�������£���100 mL 0.01 mol��L��1HA��Һ��μ���0.02 mol��L��1MOH��Һ��ͼ����ʾ���߱�ʾ�����Һ��pH�仯���������仯���Բ��ƣ����ش��������⣺

����ͼ����Ϣ��֪HAΪ_______�ᣨ�ǿ������������
�� K���Ӧ����Һ�У�
c(M��)��c(MOH)= mol��L��1��
��1���ۢܡ��ڢܢ�
��2�� 2 �� 10-3 mol��L
��3��CH3COO? + H2O CH3COOH + OH?��
CH3COOH + OH?��
c��Na+����c��CH3COO?����c��OH-����c��H+��
��4�� ��
��5���� ǿ �� 0.01
��2�� 2 �� 10-3 mol��L
��3��CH3COO? + H2O
 CH3COOH + OH?��
CH3COOH + OH?��c��Na+����c��CH3COO?����c��OH-����c��H+��
��4�� ��
��5���� ǿ �� 0.01
�����������1����HCl ��NaOH ��CH3COOH ��NH3��H2O ��CH3COONa ��NH4Cl�У� CH3COOH ��NH3��H2O����������ʣ�NaOH��NH3��H2O��CH3COONa ��Һ�Լ��ԣ���2��������0.01 mol/L HCl��Һ��PH=-lg0.01=2��PH=11��CH3COONa��Һ�е�c��H+��=10-11mol/L��CH3COONaΪǿ�������Σ�������ˮ���������c(OH��) =Kw/c��H+��=10-3 mol��L ����3��CH3COONaΪǿ�������Σ����ڴ������ˮ��ʹ������Һ�Լ��ԣ����Լ��Ե����ӷ���ʽΪCH3COO? + H2O
 CH3COOH + OH?�����ݵ���غ���Եõ�c��H+��+c��Na+��=c��OH?��+c��CH3COO?������Ϊ��Һ�Լ��ԣ�����c��OH-����c��H+��������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊc��Na+����c��CH3COO?����c��OH-����c��H+������4��HClΪǿ�ᣬ������Ϊ���ᣬ���Խ���PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���mС��n����5����ͼ����֪���� 0.01 mol��L��1HA��ҺpHΪ2������HAΪǿ��� K���Ӧ����Һ�У����������غ����֪�������MOH�����ʵ���Ϊ0.02��0.1=0.002mol������c(M��)��c(MOH)=0.002/0.2=0.01mol��L��1��
CH3COOH + OH?�����ݵ���غ���Եõ�c��H+��+c��Na+��=c��OH?��+c��CH3COO?������Ϊ��Һ�Լ��ԣ�����c��OH-����c��H+��������Һ������Ũ�Ȱ��ɴ�С��˳��Ϊc��Na+����c��CH3COO?����c��OH-����c��H+������4��HClΪǿ�ᣬ������Ϊ���ᣬ���Խ���PH�������HCl��CH3COOH�ֱ�ϡ��m����n����ϡ�ͺ�����Һ��PH����ȣ���mС��n����5����ͼ����֪���� 0.01 mol��L��1HA��ҺpHΪ2������HAΪǿ��� K���Ӧ����Һ�У����������غ����֪�������MOH�����ʵ���Ϊ0.02��0.1=0.002mol������c(M��)��c(MOH)=0.002/0.2=0.01mol��L��1�����������⿼����ǿ������ʡ�����ˮ�⡢����غ㣬�����غ�����֪ʶ����һ�����ۺ��ԣ������Ѷ����С�

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
 H����A2���ش��������⣺
H����A2���ش��������⣺ =1��10-8������������ȷ���ǣ� ����
=1��10-8������������ȷ���ǣ� ����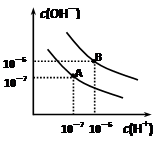
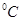 ʱˮ�ĵ���ƽ������ӦΪ ���A����B������
ʱˮ�ĵ���ƽ������ӦΪ ���A����B������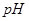 ��8��NaOH��Һ��
��8��NaOH��Һ��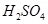 ��Һ��ϣ������û����Һ��
��Һ��ϣ������û����Һ�� ��ijǿ����Һ��1���
��ijǿ����Һ��1���