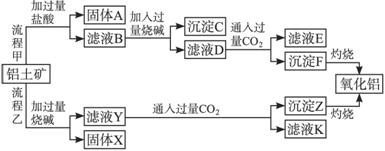
��Ŀ����
��.(1)��֪298 Kʱ2C(s)+O2(g)C(s)+O2(g)![]() CO2(g) ��H2=-393.5 kJ��mol-1
CO2(g) ��H2=-393.5 kJ��mol-1
��298 KʱCO(g)��O2(g)ȼ������CO2(g)���Ȼ�ѧ��Ӧ����ʽΪ_____________________��
(2)һ�����͵�������ȼ�ϵ�ؾ��и߷���Ч�ʶ��������ӡ�����Li2CO3��Na2CO3�������λ����������ʣ�һ��ͨ��CO���壬��һ��ͨ�������CO2�Ļ�����壬�Ƶ�ȼ�ϵ�ء�
�õ�ع���ʱ�ĸ�����ӦʽΪ______________________________��
��������![]() �����ʵ����ڹ���ʱ______________(�������С�������䡱)��
�����ʵ����ڹ���ʱ______________(�������С�������䡱)��
��.����ͼ��ʾ��AΪ��Դ��BΪ������ʳ��ˮ�ͷ�̪��Һ����ֽ����ֽ�������һ��KMnO4��Һ��C��DΪ���ۣ���缫���ϼ��������Һ��ͼ��

(1)�ر�K1����K2��ͨ���B��KMnO4�Ϻ�ɫҺ����c���ƶ������Դa��Ϊ_________����ͨ��һ��ʱ��۲쵽��ֽd�˳��ֵ�������________________��

(2)��֪Cװ������ҺΪCu(NO3)2��X(NO3)3���Ҿ�Ϊ0.1 mol����K1���ر�K2��ͨ��һ��ʱ�������������������m(g)��ͨ�����ӵ����ʵ���n(mol)��ϵ��ͼ��ʾ����Cu2+��X3+��H+���������ɴ�С��˳����___________��Dװ��Cu���ĵ缫��ӦʽΪ_________��
��.(1)CO(g)+1/2O2(g)![]() CO2(g) ��H=-283.0 kJ��mol-1
CO2(g) ��H=-283.0 kJ��mol-1
����2CO(g)+O2(g)![]() 2CO2(g) ��H=-566.0 kJ��mol-1��
2CO2(g) ��H=-566.0 kJ��mol-1��
(2)CO+![]()
![]() 2CO2+2e- ����
2CO2+2e- ����
��.(1)+(����) ���
(2)Cu2+��H+��X3+ Cu-2e-![]() Cu2+
Cu2+
��������.(2)��ع���ʱ���ܷ�Ӧ����ʽΪ��
![]()
��ͨ��CO�ļ��Ǹ�����ͨ��CO2�Ϳ�����������һ���������������Ϊ���ڵ�Li2CO3��Na2CO3���ʸ�����ӦΪ��
CO+![]()
![]() 2CO2+2e-
2CO2+2e-
���ܷ�Ӧʽ֪![]() ����������������û�б仯��
����������������û�б仯��
��.(1)�˵��Ϊ���أ�ͨ���![]() ��c���ƶ���˵��c������������Դa��Ϊ������d����������������Ӧ��2H++2e-
��c���ƶ���˵��c������������Դa��Ϊ������d����������������Ӧ��2H++2e-![]() H2��������OH-��Ũ�ȱ������̪����ɫ��
H2��������OH-��Ũ�ȱ������̪����ɫ��
(2)��ͼʾ֪ת��2 mol���Ӻ����������������䣬��0.1 mol Cu2+ת��0.2 mol����ǡ��ȫ������������Cu2+��X3+��H+�����������ɴ�С��˳���ǣ�Cu2+��H+��X3+��



 2B(g) ��H=a kJ . mol-1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ��
2B(g) ��H=a kJ . mol-1������B�����ʵ���Ũ����ʱ��仯��ͼ��ʾ�� 