��Ŀ����
X��Y��Z��W��Ԫ�����ڱ���ǰ�����ڵij���Ԫ�أ��������Ϣ���±���| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ԭ�������������Ǵ��������� |
| Z | ���ʼ��仯�������ɫ��ӦΪ��ɫ |
| W | WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
��2��X��Y�е縺�Խ�ǿ���ǣ���Ԫ�ط��ţ�______��XY2�ĵ���ʽ��______�������д���______���Ҽ���
��3��Z2Y2�к��еĻ�ѧ��������______�����������ӵĸ�����Ϊ______��
��4��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��______��
��5���Ͼ�ӡˢ��·������W�ĵ���A����H2O2��H2SO4�Ļ����Һ���ܳ�ӡˢ��·���ϵ�A����֪��
A��s��+H2SO4��aq���TASO4��aq��+H2��g����H=+64.4kJ?mol-1
2H2O2��l���T2H2O��l��+O2��g����H=-196.4kJ?mol-1
H2��g��+
 O2��g���TH2O��l����H=-285.8kJ?mol-1
O2��g���TH2O��l����H=-285.8kJ?mol-1��д��A��H2SO4��H2O2��Ӧ����ASO4��aq����H2O��l�����Ȼ�ѧ����ʽ��A�û�ѧʽ��ʾ����______��
���𰸡�������X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ���������Ų�Ϊ1s22s22p2��ΪCԪ�أ�
Yԭ�������������Ǵ�����������ӦΪOԪ�أ�
Z���ʼ��仯�������ɫ��ӦΪ��ɫ��ΪNaԪ�أ�
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ�����ӣ����������ӷֱ�Ϊ2��8��18��1��ԭ������Ϊ29��ӦΪCuԪ�أ�
���Ԫ�ض�Ӧ�ĵ��ʡ�������Ľṹ�����ʽ����⣮
����⣺X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ���������Ų�Ϊ1s22s22p2��ΪCԪ�أ�
Yԭ�������������Ǵ�����������ӦΪOԪ�أ�
Z���ʼ��仯�������ɫ��ӦΪ��ɫ��ΪNaԪ�أ�
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ�����ӣ����������ӷֱ�Ϊ2��8��18��1��ԭ������Ϊ29��ӦΪCuԪ�أ�
��1��XΪCԪ�أ�ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6��λ�����ڱ��ڶ����ڡ���A�壬���Ӧ�ĵ����н��ʯ���۵�ߡ�Ӳ�ȴ�Ϊԭ�Ӿ��壬
�ʴ�Ϊ����A��ԭ�ӣ�
��2��C��Oλ�����ڱ���ͬ���ڣ�ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����縺�Խ�ǿ��ΪO��XY2ΪCO2��Ϊ���ۻ��������ʽΪ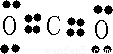 �������к���2��C=C����������2���Ҽ���
�������к���2��C=C����������2���Ҽ���
�ʴ�Ϊ��O��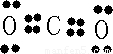 ��2��
��2��
��3��Z2Y2ΪNa2O2��Ϊ���ӻ��������O22-���������Ӽ��ͷǼ��Լ������������ӵĸ�����Ϊ1��2��
�ʴ�Ϊ�����Ӽ��ͷǼ��Լ���1��2��
��4��WΪCu��ԭ������Ϊ29����������Ų�ʽΪ1s22s22p63s23p63d104s1���ʴ�Ϊ��1s22s22p63s23p63d104s1��
��5��AΪCu����֪��Cu��s��+H2SO4��aq���TCuSO4��aq��+H2��g����H=+64.4kJ?mol-1
��2H2O2��l���T2H2O��l��+O2��g����H=-196.4kJ?mol-1
��H2��g��+ O2��g���TH2O��l����H=-285.8kJ?mol-1��
O2��g���TH2O��l����H=-285.8kJ?mol-1��
���ø�˹���ɣ�����+ ×��+�ۿɵã�Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l����
×��+�ۿɵã�Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l����
��H=��+64.4kJ?mol-1��+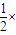 ��-196.4kJ?mol-1��+��-285.8kJ?mol-1��=-319.6 kJ/mol��
��-196.4kJ?mol-1��+��-285.8kJ?mol-1��=-319.6 kJ/mol��
�ʴ�Ϊ��Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l����H=-319.6 kJ/mol��
���������⿼��Ԫ�ص��ƶ��Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷ��еȣ������Ĺؼ��Ǹ���Ԫ��ԭ�ӽṹ������ȷ�ƶ�Ԫ�ص����࣬ע��������ӽṹ�Լ���˹���ɵ����ã�
Yԭ�������������Ǵ�����������ӦΪOԪ�أ�
Z���ʼ��仯�������ɫ��ӦΪ��ɫ��ΪNaԪ�أ�
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ�����ӣ����������ӷֱ�Ϊ2��8��18��1��ԭ������Ϊ29��ӦΪCuԪ�أ�
���Ԫ�ض�Ӧ�ĵ��ʡ�������Ľṹ�����ʽ����⣮
����⣺X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ���������Ų�Ϊ1s22s22p2��ΪCԪ�أ�
Yԭ�������������Ǵ�����������ӦΪOԪ�أ�
Z���ʼ��仯�������ɫ��ӦΪ��ɫ��ΪNaԪ�أ�
WԪ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ�����ӣ����������ӷֱ�Ϊ2��8��18��1��ԭ������Ϊ29��ӦΪCuԪ�أ�
��1��XΪCԪ�أ�ԭ�Ӻ�����2�����Ӳ㣬����������Ϊ6��λ�����ڱ��ڶ����ڡ���A�壬���Ӧ�ĵ����н��ʯ���۵�ߡ�Ӳ�ȴ�Ϊԭ�Ӿ��壬
�ʴ�Ϊ����A��ԭ�ӣ�
��2��C��Oλ�����ڱ���ͬ���ڣ�ͬ����Ԫ�ش�����Ԫ�صĵ縺������ǿ����縺�Խ�ǿ��ΪO��XY2ΪCO2��Ϊ���ۻ��������ʽΪ
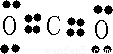 �������к���2��C=C����������2���Ҽ���
�������к���2��C=C����������2���Ҽ����ʴ�Ϊ��O��
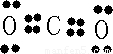 ��2��
��2����3��Z2Y2ΪNa2O2��Ϊ���ӻ��������O22-���������Ӽ��ͷǼ��Լ������������ӵĸ�����Ϊ1��2��
�ʴ�Ϊ�����Ӽ��ͷǼ��Լ���1��2��
��4��WΪCu��ԭ������Ϊ29����������Ų�ʽΪ1s22s22p63s23p63d104s1���ʴ�Ϊ��1s22s22p63s23p63d104s1��
��5��AΪCu����֪��Cu��s��+H2SO4��aq���TCuSO4��aq��+H2��g����H=+64.4kJ?mol-1
��2H2O2��l���T2H2O��l��+O2��g����H=-196.4kJ?mol-1
��H2��g��+
 O2��g���TH2O��l����H=-285.8kJ?mol-1��
O2��g���TH2O��l����H=-285.8kJ?mol-1�����ø�˹���ɣ�����+
 ×��+�ۿɵã�Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l����
×��+�ۿɵã�Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l������H=��+64.4kJ?mol-1��+
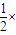 ��-196.4kJ?mol-1��+��-285.8kJ?mol-1��=-319.6 kJ/mol��
��-196.4kJ?mol-1��+��-285.8kJ?mol-1��=-319.6 kJ/mol���ʴ�Ϊ��Cu��s��+H2O2��l��+H2SO4��aq��=CuSO4��aq��+2H2O��l����H=-319.6 kJ/mol��
���������⿼��Ԫ�ص��ƶ��Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷ��еȣ������Ĺؼ��Ǹ���Ԫ��ԭ�ӽṹ������ȷ�ƶ�Ԫ�ص����࣬ע��������ӽṹ�Լ���˹���ɵ����ã�

��ϰ��ϵ�д�
�����Ŀ