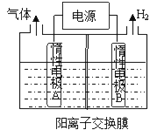
��Ŀ����
����Ŀ�����á� Na��CO2����ؽ�CO2���Ϊ�����ҹ�������Ա���Ƴ��Ŀɳ�硰 Na��CO2����������Ʋ��Ͷ��̼����(MWCNT)Ϊ�缫�������ܷ�ӦΪ4Na��3CO2 ![]() 2Na2CO3��C���ŵ�ʱ�õ�ء����롱CO2���乤��ԭ����ͼ��ʾ��
2Na2CO3��C���ŵ�ʱ�õ�ء����롱CO2���乤��ԭ����ͼ��ʾ��
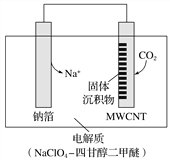
(1)�ŵ�ʱ�������ĵ缫��ӦʽΪ______________________________________________��
(2)�����ɵ�Na2CO3��Cȫ�������ڵ缫��������ת��0.2 mol e��ʱ��������������Ϊ________ g��
(3)ѡ�ø����������ĸʴ������������Һ���ŵ���___________________________________(����д����)��
���𰸡� 3CO2��4Na����4e��===2Na2CO3��C 15.8 �����Ժá�������Ʋ���Ӧ���ѻӷ���
��������(1)����CO2�õ��ӷ�����ԭ��Ӧ���ʵ缫����ʽΪ3CO2+4Na++4e-=2Na2CO3+C���ʴ�Ϊ��3CO2+4Na++4e-=2Na2CO3+C��
(2)���������ĵ缫��Ӧ3CO2+4Na++4e-=2Na2CO3+C
3 421
0.150.20.10.05��
m(��)=m(Na2CO3)+m(C)=106g/mol��0.1mol+12g/mol��0.05mol=11.2g��
���������ĵ缫��Ӧ4Na-4e-=4Na+
924
m(Na)0.2
m(Na)=4.6g����������������Ϊ11.2g+4.6g=15.8g���ʴ�Ϊ��15.8��
(3)ѡ���{������-�ĸʴ������������Һ���ŵ��ǵ����Ժã�������Ʋ���Ӧ���ѻӷ����ʴ�Ϊ�������Ժã�������Ʋ���Ӧ���ѻӷ���