��Ŀ����
�̷�(FeSO4��7H2O)���ᷨ����һ��ϡ�н�����Ʒ�����в����ĸ���Ʒ����Ʒ���Ϊ����ɫ����ɫ�ᾧ���塣���������ɵ��ڼ���ˮ�е�pH����ˮ���������л���ϣ������ٳ�������ҪӦ����ˮ�ʾ�����ҵ��ˮ������ͬʱ����ɱ�����á�
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ (��>��<��=")40%" ��
��2��ʵ��������20%��������(100�˷������ẬSO3 20��)����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200 mL 2 mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H��=6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H��=3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H��= NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS���������� (������λС��)��
��1��98% 1.84 g/cm3��Ũ������ϡ�����У��ܶ��½�����ϡ����50%ʱ���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ (������λС��)��50%��������30%������������ϣ�������Ũ��Ϊ (��>��<��=")40%" ��
��2��ʵ��������20%��������(100�˷������ẬSO3 20��)����ϡ���ᣬ����SO3��nH2O��ʾ20%�ķ������ᣬ��n=____________(������λС��)��
��3���̷��ڿ��������ױ���������Ϊ����������ȡ7.32�˾�������ϡ�������������BaCl2��Һ�����˵ó���9.32�ˣ���ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ�������Ʋ⾧��Ļ�ѧʽΪ ��
��4����������泥�(NH4)2SO4��FeSO4��6H2O��(�׳�Ī����)�����̷��ȶ����ڷ�����ѧ�г���������Fe2+�ı���Һ���ô�Fe2+�ı���Һ���Բⶨʣ��ϡ�����������ȡ8.64��Cu2S��CuS�Ļ������200 mL 2 mol/Lϡ������Һ������������Ӧ���£�
10NO3-��3Cu2S��16H��=6Cu2����10NO����3SO42-��8H2O
8NO3-��3CuS��8H��=3Cu2����3 SO42-��8NO��+ 4H2O
ʣ���ϡ����ǡ����V mL 2 mol/L (NH4)2Fe(SO4)2��Һ��ȫ��Ӧ��
��֪��NO3-��3Fe2����4H��= NO����3Fe3+��2H2O
�� Vֵ��Χ ��
�� ��V=48���Լ���������CuS���������� (������λС��)��
��1��7.14 mol��L��1 ; ��(��2��) ��2��0.77(2��)
��3��FeSO4��Fe2(SO4)3��10H2O (3��)����4����42��60mL(3��)����0.33��1/3(3��)
��3��FeSO4��Fe2(SO4)3��10H2O (3��)����4����42��60mL(3��)����0.33��1/3(3��)
�����������1���ܶ�Ϊ1.4g/cm3��50%���������ʵ���Ũ��Ϊ��
c��
 =7.14 mol��L��1��
=7.14 mol��L��1��50%��������30%����������(�����ʻ�Ϊ1���ܶȷֱ��Ǧ�1�ͦ�2)��ϣ�������Ũ��Ϊ
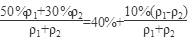 ��40%��
��40%����2����Ϊ20%��������Ϊ80��H2SO4��SO3 20�ˣ�
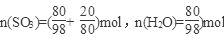 ��
������SO3��nH2O��ʾ20%�ķ������ᣬ��n=0.77��
��3��������Һ����������BaCl2��Һ�����˵ó���9.32�ˣ�n(SO42-)=
 =0.04mol����ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ����(2Fe2��+Cl2==2Fe3++2Cl-)��n(Fe2+)=2��
=0.04mol����ͨ��112mL(��״��)����ǡ�ý�Fe2����ȫ����(2Fe2��+Cl2==2Fe3++2Cl-)��n(Fe2+)=2��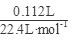 =0.01mol���ɵ���غ�n(Fe3+)=0.02mol�� 7.32�˾��庬��0.01molFeSO4Ϊ1.52g��0.01mol Fe2(SO4)3Ϊ4.00g������H2OΪ1.80g��0.1mol������Ļ�ѧʽFeSO4��Fe2(SO4)3��10H2O��
=0.01mol���ɵ���غ�n(Fe3+)=0.02mol�� 7.32�˾��庬��0.01molFeSO4Ϊ1.52g��0.01mol Fe2(SO4)3Ϊ4.00g������H2OΪ1.80g��0.1mol������Ļ�ѧʽFeSO4��Fe2(SO4)3��10H2O����4�������������ȫ��ΪCuS����n��CuS��=
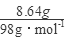 =0.09mol����Ҫ��������ʵ���Ϊy��
=0.09mol����Ҫ��������ʵ���Ϊy��8NO3-+3CuS + 8H+==3Cu2++3SO42-+8NO��+4H2O��
3 8
0.09mol y
y=0.24mol
ʣ�����������ʵ���Ϊ��0.4mol-0.24mol=0.16mol��
0.112mol����ͣ�NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��
NO3-+3Fe2++4H+==NO��+3Fe3++2H2O
3mol 4
1��10-3VL��2mol/L 0.16mol�����V=60��
����Vֵ��ΧΪ��42��V��60��
����V=48����48mL��NH4��2Fe��SO4��2��Һ��ȫ��Ӧ��Ҫ��������ʵ���Ϊ��
NO3-+3Fe2+ + 4H+��NO��+3Fe3++2H2O
3mol 4
0.048L��2mol/L n
��ã�n=0.128mol��
����������ﷴӦ����������ʵ���Ϊ��0.4mol-0.128mol=0.272mol��
��Cu2S�����ʵ���xmol��CuS�����ʵ���Ϊymol��160x+96y=8.64g�٣�
10NO3-+3Cu2S+16H+��6Cu2++10NO��+3SO42-+8H2O
3 16
x 16x/3
8NO3-+3CuS+8H+��3Cu2++3SO42-+8NO��+4H2O��
3 8
y 8y/3
16x/3+8y/3=0.272��
�ɢ٢ڽ�ã�x��0.036; y��0.03
�������CuS������������
 ��100%��33.33%��
��100%��33.33%��
��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ