��Ŀ����
.��֪���������ʹ���������Һ��ɫ����Ӧ�Ļ�ѧ����ʽΪ��5SO2+2KMnO4+2H2O====K2SO4+2MnSO4+2H2SO4��
��1��Ũ������ľ̿�ڼ��������·�Ӧ�Ļ�ѧ����ʽ��_____________________��
��2��������ͼ���и���װ�����һ��ʵ�飬����֤������Ӧ�������ĸ��ֲ����Щװ�õ�����˳��������������ҵķ�����_________![]() _________
_________![]() _________
_________![]() _________������װ�õı�ţ�
_________������װ�õı�ţ�
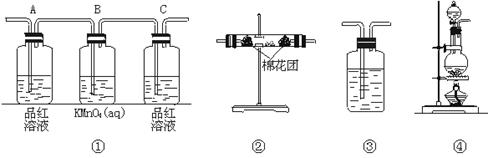
��3��ʵ��ʱ�ɹ۲쵽װ�â���Aƿ����Һ��ɫ��Cƿ����Һ����ɫ��Aƿ��Һ��������_______________��Bƿ��Һ��������______________________��Cƿ��Һ��������_______________��
��4��װ�â����ӵĹ���ҩƷ��_______________������֤�IJ�����_______________��ȷ��װ��������װ����λ�õ�������_______________��
��5��װ�â�����ʢ��Һ��_______________������֤�IJ�����_______________
��1��C+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O
CO2��+2SO2��+2H2O
��2���� �� �� ��
��3��֤��SO2�Ĵ��� ��ȥSO2 ��֤SO2�Ƿ����
��4����ˮ����ͭ���� ˮ���� ͨ��ˮ��Һ֮ǰ
��5�������ʯ��ˮ CO2
����:
Ũ�����ڼ���������������ľ̿����Ӧ�Ļ�ѧ����ʽΪC+2H2SO4��Ũ��![]() CO2��+2SO2��+2H2O��2����֤���ֲ���ʱ��Ӧ����֤H2O������ˮ����ͭ������֤SO2����Ʒ����Һ���ٳ�ȥSO2������֤CO2����װ�õ�����˳��Ϊ�ܢڢ٢ۣ�3��Aƿ��Һ����������֤SO2�Ĵ��ڡ�Bƿ��Һ�������dz�ȥSO2��Cƿ��Һ����������֤SO2�Ƿ��������4��װ�â�������֤����ˮ�Ĵ��ڣ������ӵĹ���ҩƷΪ��ˮ����ͭ���塣������ǰ������Ϊͨ������װ�ú�����ˮ�֡���5��װ�â�������֤����CO2������ʢ��Һ�dz����ʯ��ˮ��
CO2��+2SO2��+2H2O��2����֤���ֲ���ʱ��Ӧ����֤H2O������ˮ����ͭ������֤SO2����Ʒ����Һ���ٳ�ȥSO2������֤CO2����װ�õ�����˳��Ϊ�ܢڢ٢ۣ�3��Aƿ��Һ����������֤SO2�Ĵ��ڡ�Bƿ��Һ�������dz�ȥSO2��Cƿ��Һ����������֤SO2�Ƿ��������4��װ�â�������֤����ˮ�Ĵ��ڣ������ӵĹ���ҩƷΪ��ˮ����ͭ���塣������ǰ������Ϊͨ������װ�ú�����ˮ�֡���5��װ�â�������֤����CO2������ʢ��Һ�dz����ʯ��ˮ��

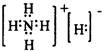
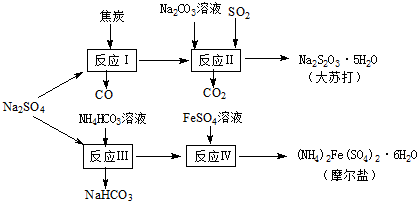 ����Ȼ�κ���Դ�з��������â�������Ṥҵ������������̿��Ϊԭ�ϣ��������մ��Ħ���Σ���ԭ�ϵ��ۺ������ʽϸߣ�����Ҫ�������£�
����Ȼ�κ���Դ�з��������â�������Ṥҵ������������̿��Ϊԭ�ϣ��������մ��Ħ���Σ���ԭ�ϵ��ۺ������ʽϸߣ�����Ҫ�������£�
 ��ȡ��ƿ����Һ���������Ʒ����Һ�������ʵ������Ϊ
��ȡ��ƿ����Һ���������Ʒ����Һ�������ʵ������Ϊ