��Ŀ����
��6�֣�������Ԫ��A��B��C��Dԭ��������������A�ĵ�������������壬A��Cͬ���壬ֻ��C�ǽ���Ԫ�أ�B�������������Ǵ�����������3����B��C������������֮����D��������������ȡ�
��1��C��ԭ�ӽṹʾ��ͼΪ ��
��2��������C2B2�����������Ӹ�����Ϊ ��
��3��������CDB�Ǽ�������������Ҫ�ɷ֣�����������Ϊ ��
��4����CD��Һ�еμ���������Һ,�ɹ۲쵽�������� ���䷴Ӧ�����ӷ���ʽΪ ��
���𰸡�
��1��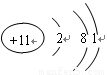 ��1�֣�
��1�֣�
��2��1: 2 �����֣� ��3���������� �����֣�
��4����ɫ���� �����֣� Ag+ +Cl- =AgCl�� ��2�֣�
��������

��ϰ��ϵ�д�
�����Ŀ