��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣���Ԫ�ط��Ż�ѧʽ�ش��������⡣
���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
2 | �� | �� | ||||||
3 | �� | �� | �� | �� | �� | |||
4 | �� | �� | �� |
��1��10��Ԫ���У���ѧ��������õ���_________��������ԭ�Ӱ뾶������________��
��2���٢ۢ�����Ԫ������������Ӧ��ˮ���������ǿ����__________ ������γ��⻯����ȶ�����ǿ����˳��Ϊ_________________��
��3���٢ڢۢݢ�����Ԫ���γɵ����Ӱ뾶�ɴ�С��˳��Ϊ______________________
��4��Ԫ�آ�������____��Ԫ�آܵ�ԭ�ӽṹʾ��ͼ��/span>_______��Ԫ�آߵ��⻯���Ԫ�آٵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ________________��
��5���٢��γɵĻ���������ʱ������ɫΪ________���û��������Һ��Ԫ�آ�ĵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ_______________________��
��6���١��ݺ͢�����������Ӧ��ˮ����֮���������Ӧ�����ӷ���ʽΪ____________________��
��7��Ԫ�آ۵ĵ�����Ԫ�آޢ��γɵ�ij��������ȼ�յĻ�ѧ����ʽΪ____________________��
���𰸡� Ar K NaOH HCl>HBr Cl->K+>Na+>Mg2+>Al3+ ̼ 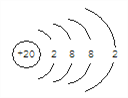 2Na+2H2O==2NaOH+H2�� ��ɫ Cl2+2NaBr==Br2+2NaCl H++OH-==H2O Al(OH)3+3H+==Al3++3H2O Al(OH)3+OH-==AlO2-+2H2O 2Mg+CO2
2Na+2H2O==2NaOH+H2�� ��ɫ Cl2+2NaBr==Br2+2NaCl H++OH-==H2O Al(OH)3+3H+==Al3++3H2O Al(OH)3+OH-==AlO2-+2H2O 2Mg+CO2![]() 2MgO+C
2MgO+C
������������Ԫ�������ڱ��е�λ�ÿ��ж����Ƿֱ���Na��K��Mg��Ca��Al��C��O��Cl��Br��Ar����
��1��10��Ԫ���У���ѧ��������õ���ϡ������Ar��������ԭ�Ӱ뾶������K����2��ͬ�����������ҽ�������������٢ۢ�����Ԫ������������Ӧ��ˮ���������ǿ����NaOH��ͬ������ϵ��·ǽ����������������γ��⻯����ȶ�����ǿ����˳��ΪHCl>HBr����3�����ӵĺ�����Ӳ���Խ�࣬���Ӱ뾶Խ��������Ų���ͬʱ�����Ӱ뾶��ԭ���������������С����٢ڢۢݢ�����Ԫ���γɵ����Ӱ뾶�ɴ�С��˳��ΪCl->K+>Na+>Mg2+>Al3+����4��Ԫ�آ�������̼��Ԫ�آܵĺ����������20��ԭ�ӽṹʾ��ͼ��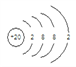 ��Ԫ�آߵ��⻯���Ԫ�آٵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O��2NaOH+H2������5������Ԫ�صĻ���������ʱ����Ϊ��ɫ������������ǿ���壬���������廯�Ʒ�Ӧ�Ļ�ѧ����ʽΪCl2+2NaBr==Br2+2NaCl����6�������ݺ͢�����������Ӧ��ˮ����ֱ����������ơ��������������ᣬ֮���������Ӧ�����ӷ���ʽΪ�ֱ���H++OH-==H2O��Al(OH)3+3H+==Al3++3H2O��Al(OH)3+OH-==AlO2-+2H2O����7��Ԫþ��CO2��ȼ�յĻ�ѧ����ʽΪ2Mg+CO2
��Ԫ�آߵ��⻯���Ԫ�آٵ��ʷ�Ӧ�Ļ�ѧ����ʽΪ2Na+2H2O��2NaOH+H2������5������Ԫ�صĻ���������ʱ����Ϊ��ɫ������������ǿ���壬���������廯�Ʒ�Ӧ�Ļ�ѧ����ʽΪCl2+2NaBr==Br2+2NaCl����6�������ݺ͢�����������Ӧ��ˮ����ֱ����������ơ��������������ᣬ֮���������Ӧ�����ӷ���ʽΪ�ֱ���H++OH-==H2O��Al(OH)3+3H+==Al3++3H2O��Al(OH)3+OH-==AlO2-+2H2O����7��Ԫþ��CO2��ȼ�յĻ�ѧ����ʽΪ2Mg+CO2![]() 2MgO+C��
2MgO+C��

����Ŀ��Ϊ��ȥij���������������ʣ���ѡ�õ��Լ������������ȷ����( )
��� | ���� | ���� | �����Լ���������� |
�� | NaCl��Һ | Na2CO3 | �������ᣬ���� |
�� | FeSO4��Һ | CuSO4 | ����������۲����� |
�� | H2 | CO2 | ����ͨ��ʢ��NaOH��Һ��Ũ�����ϴ��ƿ |
�� | NaNO3 | CaCO3 | ��ϡ�����ܽ⡢���ˡ��������ᾧ |
A.�٢ڢ� B���ڢۢ� C���٢ۢ� D���٢ڢۢ�
����Ŀ��X��Y��Z��P��Q��W��R��T��Ԫ�����ڱ����������������Ԫ�أ���ԭ���������������������Ϣ���±���
Ԫ�� | �����Ϣ |
X | ��ijһ��ͬλ�أ����������ԭ�������ı� |
Y | ������������Ӧˮ������������̬�⻯������Ϸ�Ӧ |
P | �Ƕ������У���ϡ�������⣩ԭ�Ӱ뾶����Ԫ�� |
Q | ����������Ϊ25��������Ϊ13�ĺ��� |
W | λ�����ڱ���13�� |
R | ��Zͬ�壬 ������������Ӧˮ�����Ũ��Һ��������W ���ʻ���ֶۻ����� |
(1)WԪ��λ�����ڱ���________����______�壻T���ӵĽṹʾ��ͼΪ____________��
(2)��Q��Z��R�ļ����ӵİ뾶�Ӵ�С����___________(�����ӷ��ű�ʾ����R��T����Ԫ�ص�����������Ӧˮ������ᣨ������ǿ������˳��Ϊ__________(�û�ѧʽ��ʾ)��
(3) X��Y��Z����Ԫ�صļ��⻯���У��ȶ�����ǿ�Ļ�����ĵ���ʽΪ_________��������P2R��Һ�ڿ����г��ڷ��ã����������Ӧ������������ƵĽṹ���Ƶ�����P2R2������Һ�Ի�ɫ����P2R2�ĵ���ʽΪ______________��
(4)�����±��е���Ϣ���ж����л������̬ʱ�ľ������ͣ�����Ӿ��塱�����ȣ���
������ | x3Y4 | R2T2 |
��Ҫ�������� | Ӳ�ȿ��Ժͽ��ʯ���������۵�>3600�� | ��������һ�ֳȻ�ɫҺ�壬�۵�Ϊ-76��C |
�������� | ________ | _________ |
(5)Q�ĵ�������X��Z�γɵ�ij�ֻ����ﷴӦ��д���仯ѧ����ʽ��______________��
(6)X��Q�γɵ�lmol������Q2X3��ˮ��Ӧ������2molMg(OH)2��1molij��̬��������������̼��������Ϊ9: 1��д������̬���Ľṹ��ʽ_______����д��Q2X3��ˮ��Ӧ�Ļ�ѧ����ʽ________��

