��Ŀ����
��֪�¶�Խ�ߣ�AgI��ˮ�е��ܽ��Խ��������AgI�����AgI������Һ�зֱ�������²������й�����Ũ�ȵı仯����ǣ�
��1������������ˮ����c��I-��______�����������С�����䡱��
��2��������AgNO3���壬��AgI��ˮ�е��ܽ��______�����������С�����䡱��
��3��������NaCl���壬��AgI���ܶȻ�Ksp______�����������С�����䡱��
��4����ij�¶��£�AgI���ܶȻ�Ksp=9.0��10-20����AgI�ڸ��¶��µ��ܽ����______g��AgI������Һ���ܶȿɽ���Ϊ1g?mL-1����
��1������������ˮ����c��I-��______�����������С�����䡱��
��2��������AgNO3���壬��AgI��ˮ�е��ܽ��______�����������С�����䡱��
��3��������NaCl���壬��AgI���ܶȻ�Ksp______�����������С�����䡱��
��4����ij�¶��£�AgI���ܶȻ�Ksp=9.0��10-20����AgI�ڸ��¶��µ��ܽ����______g��AgI������Һ���ܶȿɽ���Ϊ1g?mL-1����
��һ���¶��£���AgI�ı�����Һ�д��ڳ����ܽ�ƽ�⣬AgI��s��?Ag+��aq��+I-��aq����ƽ��״̬�´����ܶȻ�������Ksp=C��Ag+��?C��I-����
��1������������ˮ�������ܽ⣬�����γɱ�����Һ����������Ũ�Ȳ��䣬�ʴ�Ϊ�����䣻
��2��������AgNO3���壬������������Ũ�ȣ������ܽ�ƽ�������ƶ���c��I-����С���ʴ�Ϊ����С��
��3��������NaCl���壬�����������Ũ�ȣ������ܽ�ƽ�������ƶ���c��I-�������ܶȻ�Ksp���䣻�ʴ�Ϊ�����䣻
��4��AgI���ܶȻ�Ksp=9.0��10-20����C��Ag+��=3��10-10mol/L������Һ�����Ϊ1L��n��AgI��=n��Ag+��=3��10-10mol/L��1L=3��10-10mol���ܽ��AgI������Ϊ3��10-10mol��235g/mol=7.05��10-8g����Һ������Ϊ��1000ml��1g?mL-1=1000g��ˮ������Ϊ1000g-7.05��10-8g=1000g��AgI�ڸ��¶��µ��ܽ����
=7.05��10-9g���ʴ�Ϊ��7.05��10-9��
��1������������ˮ�������ܽ⣬�����γɱ�����Һ����������Ũ�Ȳ��䣬�ʴ�Ϊ�����䣻
��2��������AgNO3���壬������������Ũ�ȣ������ܽ�ƽ�������ƶ���c��I-����С���ʴ�Ϊ����С��
��3��������NaCl���壬�����������Ũ�ȣ������ܽ�ƽ�������ƶ���c��I-�������ܶȻ�Ksp���䣻�ʴ�Ϊ�����䣻
��4��AgI���ܶȻ�Ksp=9.0��10-20����C��Ag+��=3��10-10mol/L������Һ�����Ϊ1L��n��AgI��=n��Ag+��=3��10-10mol/L��1L=3��10-10mol���ܽ��AgI������Ϊ3��10-10mol��235g/mol=7.05��10-8g����Һ������Ϊ��1000ml��1g?mL-1=1000g��ˮ������Ϊ1000g-7.05��10-8g=1000g��AgI�ڸ��¶��µ��ܽ����
| 7.05��10-8g��100 |
| 1000 |

��ϰ��ϵ�д�
�����Ŀ
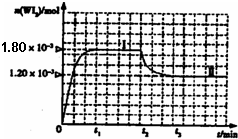 ��2010?��ׯһģ��±�ص��ʵ����ʻ��ã�±�صĻ�����Ӧ�ù㷺���о�±�ص��ʼ��仯���������Ҫ���壮
��2010?��ׯһģ��±�ص��ʵ����ʻ��ã�±�صĻ�����Ӧ�ù㷺���о�±�ص��ʼ��仯���������Ҫ���壮

 WI2(g)��Ϊģ��������Ӧ��ȷ��ȡ0.508g�⡢0.736g����������50.0mL���ܱ������У�����ʹ�䷴Ӧ����ͼ��WI2��g�������ʵ�����ʱ��仯��ϵͼ����������I��0~t2ʱ��Σ��ķ�Ӧ�¶�ΪT1������II����t2��ʼ���ķ�Ӧ�¶�ΪT2����T2>T1���ٸ÷�Ӧ�ġ�H 0������ڡ�
WI2(g)��Ϊģ��������Ӧ��ȷ��ȡ0.508g�⡢0.736g����������50.0mL���ܱ������У�����ʹ�䷴Ӧ����ͼ��WI2��g�������ʵ�����ʱ��仯��ϵͼ����������I��0~t2ʱ��Σ��ķ�Ӧ�¶�ΪT1������II����t2��ʼ���ķ�Ӧ�¶�ΪT2����T2>T1���ٸ÷�Ӧ�ġ�H 0������ڡ�
