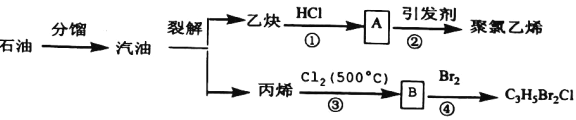
��Ŀ����
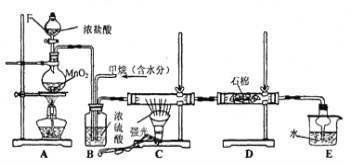
����Ŀ�������Ͱ����ڳ����»�ϼ��ɷ�����Ӧ��ij��ȤС��ͬѧΪ̽������������������백���ķ�Ӧ�����������װ�ã�
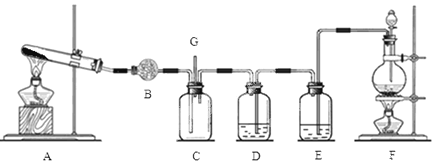
��1��װ��F�з�����Ӧ�����ӷ���ʽ�� ��
��2��װ��B��ʢ�ŵ��� ���������� ��װ��E��ʢ�ŵ��Լ��� ���������� ��
��3��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ�� ��
��4������10.7gNH4Cl��������ȡ��״���µİ���������� L��
��5��װ��C���а��̲�������д������Ӧ�Ļ�ѧ����ʽ
��6��G���ݳ���β���к�������Cl2��Ϊ��ֹ����Ⱦ�������ɽ�β��ͨ��ʢ�� ��ϴ��ƿ��
���𰸡���1)MnO2 + 4H+ + 2Cl-![]() Mn2+ + Cl2��+ 2H2O��
Mn2+ + Cl2��+ 2H2O��
��2����ʯ�ң����ﰱ��������ʳ��ˮ������HCl���壻
��3��Ca��OH��2+2NH4Cl![]() CaCl2+2NH3��+2H2O ��4��4.48��
CaCl2+2NH3��+2H2O ��4��4.48��
��5��NH3+HCl��NH4Cl����6��NaOH��Һ
��������
�����������1��װ��F��Ũ������������̻�ϼ�����ȡ������װ�ã����з�����Ӧ�����ӷ���ʽ��MnO2 + 4H+ + 2Cl-![]() Mn2+ + Cl2��+ 2H2O��
Mn2+ + Cl2��+ 2H2O��
��2�������İ����к���ˮ��������Bװ���������dz�ȥˮ���������ﰱ���������Լ��Ǽ�ʯ��������Ũ������лӷ��ԣ�������ȡ�������к�������HCl��ˮ��������װ��E���ñ���ʳ��ˮ��ȥHCl����װ��D����Ũ�����ȥ���е�ˮ������
��3���� Aװ����������ȡ�������÷�Ӧ�ķ���ʽ�ǣ�Ca(OH)2+2NH4Cl![]() 2NH3��+ 2H2O+CaCl2��
2NH3��+ 2H2O+CaCl2��
��4��10.7g NH4Cl�����ʵ�����n(NH4Cl)=10.7g��53.5g/mol=0.2mol������ݷ���ʽ��֪��Ӧ�������������ʵ���Ҳ��0.2mol�����ڱ�״���µ������V(NH3)=0.2mol��22.4 L/mol��4.48L��
��5������C���а��̲�����˵�����Ȼ�鱗���������Ӧ�Ļ�ѧ����ʽ��NH3+HCl==NH4Cl��
��6��G���ݳ���β���к�������Cl2����������������Ӧ�����������Ե��Σ�����Ϊ��ֹ����Ⱦ�������ɽ�β��ͨ��ʢ��NaOH��Һ��ϴ��ƿ��