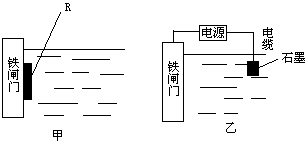
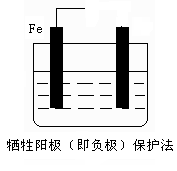
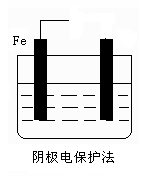
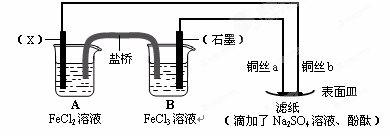
��Ŀ����
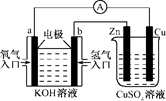
��8�֣����ϵ���ǽ���ʮ������չ�����ı��洦���¼������㷺���ڵ��ӡ����õ����������������ȹ�ҵ�������������ϡ��մɵȷǽ���������û�ѧ�����ķ���������һ������㣬���õ��ķ�������ͭ�㣬���ҺΪ CuSO4ˮ��Һ��
��1����д�����ʱ�������Ͽ��ܷ����ķ�Ӧ��
������ ����������
�������� �� ��������
��2����ͨ��0.01mol���Ӻ������ϳ�����ͭ 0.2879g , �����ϻ����ܷų��������� �������״�������������ų��������� ��
��1����д�����ʱ�������Ͽ��ܷ����ķ�Ӧ��
������ ����������
�������� �� ��������
��2����ͨ��0.01mol���Ӻ������ϳ�����ͭ 0.2879g , �����ϻ����ܷų��������� �������״�������������ų��������� ��
��1���� Cu2++ 2e- = Cu���ڡ���2H++2e- =H2 ���� a. H2 �� 0.0672L
��1�������õ����ӣ����Ը������ӵķŵ�˳���֪��������ͭ���ӷŵ磬Ȼ���������ӷŵ磬����ʽ�ֱ���Cu2++ 2e- = Cu��2H++2e- =H2 ����
��2��0.2879gͭ��0.0045mol���õ�������0.009mol�����Ը��ݵ��ӵ��غ��֪������������0.0005mol����״���µ������11.2ml����������Һ�е�OH���ŵ��������������ʵ�����0.0025mol����״���µ������56ml�������������11.2ml��56ml��67.2ml��
��2��0.2879gͭ��0.0045mol���õ�������0.009mol�����Ը��ݵ��ӵ��غ��֪������������0.0005mol����״���µ������11.2ml����������Һ�е�OH���ŵ��������������ʵ�����0.0025mol����״���µ������56ml�������������11.2ml��56ml��67.2ml��

��ϰ��ϵ�д�
 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д� ��У���˿��ֿ���ϵ�д�
��У���˿��ֿ���ϵ�д�
�����Ŀ
 LiFePO4������еĹ������ʿɴ���Li+����õ�س��ʱ�����缫��ӦʽΪ��LiFePO4-e-=FePO4+Li+
LiFePO4������еĹ������ʿɴ���Li+����õ�س��ʱ�����缫��ӦʽΪ��LiFePO4-e-=FePO4+Li+