��Ŀ����
�й��������жϻ��ʾ������ȷ���� ( )
| A���ɣ�H+(aq) + OH��(aq)= H2O(l)��H =��57��3kJ/mol����֪����1mol CH3COOH����Һ�뺬1mol NaOH����Һ��ϣ��ų���������57��3 kJ |
| B�����������������������ֱ���ȫȼ�գ����߷ų��������� |
| C����C��ʯī��= C�����ʯ����H =" +" 1��9kJ/mol����֪ʯī�Ƚ��ʯ���ȶ� |
| D��2gH2��ȫȼ������Һ̬ˮ�ų�285��8kJ������������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2(g)+O2(g)="2" H2O(l)��H =��285��8kJ/mol |
C
�������������A��CH3COOH�����ᣬ������Ҫ���ȣ����Ժ�1mol CH3COOH����Һ�뺬1mol NaOH����Һ��ϣ��ų���������57��3 kJ������B�����������������������ֺ��е�����ǰ�߸ߣ��ֱ���ȫȼ�գ�������ͬ״̬��������ʱ�ų�����ǰ�߸��ࡣ����C�����������е�����Խ��Խ�ȶ������ڴ�C��ʯī��= C�����ʯ����H =" +" 1��9kJ/mol���������������Կ�֪ʯī�Ƚ��ʯ���ȶ�����ȷ��D��������ȼ�յ��Ȼ�ѧ����ʽΪ��2H2(g) + O2(g) = 2 H2O(l) ��H =��571��6kJ/mol������
���㣺�����й��������жϻ��ʾ������֪ʶ��

 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�����ͼ��ʾ����H1=��393.5 kJ?mol-1����H2=��395.4 kJ?mol-1������˵�����ʾʽ��ȷ����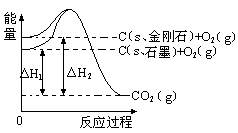
| A��ʯī�ͽ��ʯ��ת���������仯 |
| B��1molʯī����������1 mol���ʯ����������1.9 kJ |
| C�����ʯ���ȶ���ǿ��ʯī |
| D��C(s��ʯī)=C(s�����ʯ) ��H=" +1.9" kJ?mol-1 |
һ�������£����ȼ��һ�����Ķ���ų�QkJ�����������ⶨ��ȫ�������ɵĶ�����̼������5 mol��L-1��KOH��Һ100 mL��ǡ���������Ρ���������·�ӦC4H10(g)+13/2O2(g) = 4CO2(g)+5H2O(g)�Ħ�HΪ�� ��
| A��+8Q kJ��mol-1 | B��+16Q kJ��mol-1 | C����8Q kJ��mol-1 | D����16Q kJ��mol-1 |
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
| A����֪2H2(g)��O2(g)��2H2O(g)����H����483.6 kJ·mol-1,��������ȼ����Ϊ241.8 kJ·mol-1 |
| B����֪NaOH(aq)��HCl(aq)��NaCl(aq)��H2O(l)����H����57.3 kJ·mol-1 ��40.0g NaOH��ϡ��Һ��ϡ������ȫ�кͣ��ų�57.3kJ������ |
| C����֪C(ʯī��s)��C(���ʯ��s)����H��0����ʯī�Ƚ��ʯ�ȶ� |
| D����֪2C(s)��2O2(g)��2CO2(g)������H=a��2C(s)��O2(g)��2CO(g)����H=b����a��b |
��(H2NNH2)��һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯����ͼ��ʾ����֪����1 mol��ѧ�����������(kJ)��N��NΪ942��O=OΪ500��N��NΪ154�������1 mol N��H����������(kJ)��(����)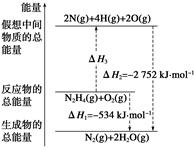
| A��194 | B��391 | C��516 | D��658 |
�����뻯ѧ��Ӧ�����仯��ص�������ȷ����
| A����Ӧ���������һ������������������� |
| B��������������Ӧ����������ԭ��Ӧ���������ȷ�Ӧ |
| C����ѧ��Ӧ�з��������仯�ĸ���ԭ���ǻ�ѧ���Ķ������γ� |
| D��ͬ��ͬѹ�£�H2(g)+Cl2(g)=2HCl(g)�ڹ��պ͵�ȼ�����µġ�H��ͬ |
�����з�Ӧ�����������������ڷ�Ӧ������������
| A������������ˮ | B���Ҵ�ȼ�� | C����������������ĩ��Ӧ | D��̼������ȷֽ� |
���������Ȼ�ѧ����ʽ�ó��Ľ�����ȷ���ǣ� ��
A����֪2SO2(g)+O2 2SO3(g)Ϊ���ȷ�Ӧ����SO2������һ������SO3������ 2SO3(g)Ϊ���ȷ�Ӧ����SO2������һ������SO3������ |
| B����֪C(ʯī,s)=C�����ʯ,s����H��0������ʯ��ʯī�ȶ� |
| C����֪H+(aq)+OH-(aq)=H2O��1����H=��57��3kJ��mol�����κ�����к͵ķ�Ӧ�Ⱦ�Ϊ57��3kJ |
| D����֪2C(s)+2O2(g)=2CO2(g)��H1 |