��Ŀ����
����Ŀ������˵����ȷ����
A. ʵ������Zn��ϡ������H2��Ϊ�ӿ췴Ӧ���ʣ�����ϡ�����м�������Cu��
B. CH3Cl(g)��Cl2(g)![]() CH2Cl2(l)��HCl(g)���������Է����У���÷�Ӧ�Ħ�H>0
CH2Cl2(l)��HCl(g)���������Է����У���÷�Ӧ�Ħ�H>0
C. ��һ����ɱ�ĺ����ܱ������з�����ӦPCl3(g)��Cl2(g) ![]() PCl5(g)��ѹ�������ƽ�������ƶ���Kֵ����
PCl5(g)��ѹ�������ƽ�������ƶ���Kֵ����
D. 25��ʱKa(HClO)��3.0��10��8��Ka(HCN)��4.9��10��10�������¶���NaClO��Һ��NaCN��ҺpH��ͬ����c(NaClO)<c(NaCN)
���𰸡�A
��������A��ϡ�����м�������Cu�ۣ�Zn-H2SO4-Cu����ԭ��أ����Լӿ췴Ӧ���ʣ�A��ȷ��B��CH3Cl(g)��Cl2(g)![]() CH2Cl2(l)��HCl(g)���ر�С��0�����������Է����У����ݸ����оݿ�֪�÷�Ӧ����Hһ��С��0��B����C��������ѹ������൱������ѹǿ���÷�Ӧ��ƽ�������ƶ����¶Ȳ��䣬Kֵ���䣬C����D��Ka(HClO)>Ka(HCN)��HClO�����Դ���HCN�����ԣ�������ͬŨ�ȵ�ClO-��ˮ��̶�С��CN-��ˮ��̶ȣ�ǰ��ˮ��õ���OH-Ũ��С�ں���ˮ��õ���OH-Ũ�ȣ�Ҫʹ����ˮ��õ���OH-Ũ����ͬ����NaClO��Ũ��Ҫ����NaCN��Ũ�ȣ�D������ȷ��ΪA��
CH2Cl2(l)��HCl(g)���ر�С��0�����������Է����У����ݸ����оݿ�֪�÷�Ӧ����Hһ��С��0��B����C��������ѹ������൱������ѹǿ���÷�Ӧ��ƽ�������ƶ����¶Ȳ��䣬Kֵ���䣬C����D��Ka(HClO)>Ka(HCN)��HClO�����Դ���HCN�����ԣ�������ͬŨ�ȵ�ClO-��ˮ��̶�С��CN-��ˮ��̶ȣ�ǰ��ˮ��õ���OH-Ũ��С�ں���ˮ��õ���OH-Ũ�ȣ�Ҫʹ����ˮ��õ���OH-Ũ����ͬ����NaClO��Ũ��Ҫ����NaCN��Ũ�ȣ�D������ȷ��ΪA��

 һ��һ����ʱ���ϵ�д�
һ��һ����ʱ���ϵ�д�����Ŀ��ijѧϰС��Ϊ��̽��Fe(NO3)3�����ȶ��Ժ������ԣ��������ʵ�飺
ʵ��(һ)�� ���ȶ���
������ͼװ�ý���ʵ�飬����A��Fe(NO3)3���壬A ��B�ж��к���ɫ���������A �Թ��������˺�ɫ��ĩ��
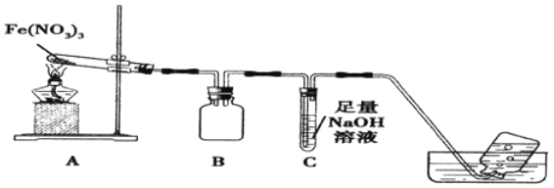
��1��Bװ�õ�����____________��Ҫ����A�к�ɫ�����Ƿ�ΪFe2O3��Ӧ��ѡ�õ��Լ���____________(�ѧʽ)��
��2������A���Թ�һ��ʱ���C �е��ܿ������ݲ�����������ƿ�������ݲ���ԭ����______________��
��3��д�����������ȷֽ�Ļ�ѧ����ʽ__________________��
ʵ��(��)��������
Fe3+��Ag+�����������ǿ��һֱ��ʵ��̽�����ȵ㡣��С���������ʵ�飺
������ | ʵ����� | ���� |
a | ��10mL3mol��L-1KNO3������Һ(pH=1)�в���һ���ྻ����˿�����μ��Ȼ�����Һ | �������� |
b | ��10mL3mol��L-1AgNO3��Һ�еμ�2mL0.1mol��L-1FeSO4��Һ�������ٵμ����Ը��������Һ | �Ϻ�ɫ��Һ����ɫ |
c | ��10 mL3mol��L-1Fe(NO3)3��Һ(pH=1)�в���һ���ྻ����˿�����μ��Ȼ�����Һ | ������ɫ���� |
��4�����ʵ��a��Ŀ����_____________________��ʵ��c�Ľ�����_____________________________��
��5��ʵ��b�漰��Ӧ�����ӷ���ʽΪ____________________________________��
��6��ʵ�������Fe3+��Ag+�����Ե����ǿ�������ӵ�_____________________�й���

