��Ŀ����
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺

��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���____________(�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)����ԭ����_______________________________________________��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H��______ __�����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ_______________________________��

��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���____________(�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)����ԭ����_______________________________________________��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
��֪���ᡢNaOH��Һ�ܶȽ���Ϊ1.00g/cm3���кͺ���Һ�ı�����c��4.18��10��3kJ/(g����)����÷�Ӧ���к���Ϊ��H��______ __�����ݼ�������д�����кͷ�Ӧ���Ȼ�ѧ����ʽ_______________________________��
��ƫС����ƫС����ƫС���ô���������ᣬ�������Ҫ������������ɲ�õ��к���ƫС��
�ȣ�56.01 kJ/mol��HCl(aq)��NaOH(aq)��NaCl(aq)��H2O(l) ��H����56.01 kJ/mol
�����������1������Ϊ�з������������������ڷ�Ӧ�лӷ���������HCl�������ʲ�õ��к��Ȼ�ƫС��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬��Ŀ���Ƿ�ֹ������IJ�Һ���������Ʒ�Ӧ�����˲������裬��ʹ�ò�ý��ƫС��
��3��ƫС���ô���������ᣬ�������Ҫ������������ɲ�õ��к���ƫС��
��4���²�(t2��t1)Ӧȡ����ʵ���ƽ��ֵ6.7 �������㡣
��H����
 ����
����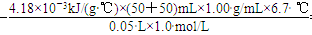 ����56.01 kJ/mol��
����56.01 kJ/mol��HCl(aq)��NaOH(aq)��NaCl(aq)��H2O(l) ��H����56.01 kJ/mol

��ϰ��ϵ�д�
�����Ŀ


 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
 Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ
Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ

 2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
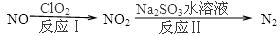

 C2H4(g)����H2����Q2
C2H4(g)����H2����Q2





 H2(g)+
H2(g)+
 2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻
2NH3(g)��H<0�仯ѧƽ�ⳣ��K���¶�t�Ĺ�ϵ���ұ���������������⣻