��Ŀ����
��15�֣�����������һ�ְ�ɫ�й���Ƭ״�ᾧ���ɫ�ᾧ��ĩ���ǻǰ���ҩ���ԭ�ϣ�������ֹʹ�������ȼ�����������Ⱦ���м��塣
�����������Ʊ�ԭ��Ϊ�� 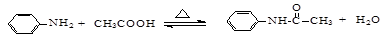
ʵ�������
| ���� | ʽ�� | ��״ | �ܶ�/g/cm3 | �е�/�� | �ܽ�� | |
| �� �� | 93 | ��ɫ��״Һ�壬 ���л�ԭ�� | 1.02 | 184.4 | ����ˮ | �������Ҵ������ѵ� |
| �� �� | 60 | ��ɫҺ�� | 1.05 | 118.1 | ������ˮ | �������Ҵ������� |
| �������� | 135 | ��ɫ���� | 1.22 | 304 | ������ˮ��������ˮ | �������Ҵ������� |
ע�����η������������൱�ڶ����������ڷе���̫��Ļ����ķ���
ʵ�鲽�裺
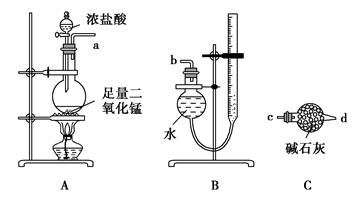
����1����100mlԲ����ƿ�м�����ˮ����9.1mL��������17.4mL��п��0.1g����װ�����������ʯ������Ӧ�����ȼ��ȣ�ʹ��ӦҺ����״̬�»�����

���ڼ����¶ȣ�ʹ�����¶ȿ�����105�����ң���ӦԼ60��80min����Ӧ���ɵ�ˮ���������ᱻ����������Ӧ�������ʱ��ֹͣ���ȡ�
����2�� �ڽ����£����Ƚ���ƿ�е�������ϸ��״����ʢ��100mL��ˮ���ձ��У����ҽ��裬����ȴ�ձ������£������������ᾧ���������ˡ�ϴ�ӡ�����õ�����������Ʒ��
����3�� ���˴��������������ؽᾧ�����ᾧ��ȫ����ˣ�����ѹ���˱�����Ʒ���ڸɾ��ı����������ɣ����أ�������ʡ�
��ش��������⣺
��1��ʵ���м���п�۵�Ŀ����
��2��Ϊ��Ҫ���Ʒ������϶˵��¶���105?C����
��3��ͨ��ʲô������жϷ�Ӧ�������
��4������1���ȿ��� ���ˮԡ������ԡ����������2�нᾧʱ������ȴ���������������ɲ��õķ����� ��
��5��ϴ������������Ʒ����ʵ��Լ��� ��
A. ��������ˮϴ B. ��������ˮϴ
C. ������ˮϴ��������ˮϴ D. �þƾ�ϴ
��6����ʵ�����յõ���Ʒ8.1�ˣ������������IJ����� ��
��1����ֹ�����ڷ�Ӧ�����б�������
��2�����Ϸֳ���Ӧ���������ɵ�ˮ���ٽ���Ӧ�������
��3���¶ȼ��¶��½�����ƿ��Һ�岻�����ӣ�
��4����ԡ���ò�����Ħ�������ڱڣ���Ͷ�뾧�ֻ��ñ�ˮ��ȴ����
��5��A ����6��60%
���������������1���������ȶ��������ڿ����е���������Ϊ��������Ϊ��ֹ�����ڷ�Ӧ�����б����������뻹ԭ��Zn�ۡ���2��ˮ�ķе���100�棬������105�����ң��Ϳ��Բ��Ϸֳ���Ӧ���������ɵ�ˮ���ٽ���Ӧ������У����������IJ��ʡ���3���������¶ȼ��¶��½�����ƿ��Һ�岻�����Ӿͱ��淴Ӧ����������٣�������ﵽ�����������Ӧ������ɣ���4����4�����ڲ���1���ȵ��¶���105�����ң������ڵ�ˮ�¶�Ϊ100�棬���ܴﵽ���¶ȣ����Բ���1������ԡ���ȵķ���������2�нᾧʱ������ȴ���������������ɲ��õķ������ò�����Ħ�������ڱڣ���Ͷ�뾧�ֻ��ñ�ˮ��ȴ���ķ���ʵ�ֽᾧ����5��������������������ˮ��������ˮ������ϴ�Ӵ�Ʒ����ʵ��Լ�����������ˮϴ���Լ�����ϴ����ɵ���ġ���ѡ��ΪA����6��n(����)=" (9.1mL" ��1.02g/ml)��93g/mol=0.1mol��n(����)=( 17.4mL ��1.05g/ml)��60g/mol=0.305mol�����ڱ��������ʵ��������Բ����������������ʵ���Ҫ�Բ������ı���Ϊԭ��Ϊ�������۲���Ϊ0.1mol����ʵ�ʲ���n(��������)= 8.1g��135g/mol=0.06mol���������������IJ���Ϊ0.06mol��0.1mol��100%=60%��
���㣺�������ʵ��Ʊ��������Ŀ��Ƽ�ʵ���������Ӧ�̶ȵ��жϡ����ʵIJ��ʵļ����֪ʶ��

����ʵ���ܴﵽԤ��Ŀ�ĵ���
| A����ͼ����֤ͭ��ϡ���ᷴӦ���������ֻ��NO |
| B����ͼ��ʵ������ȡ�������� |
| C����ͼ����֤����Ƭ����������ʴ |
| D����ͼ��֤������ͬ�¶���Mg(OH)2���ܽ�ȴ���Fe(OH)3 |
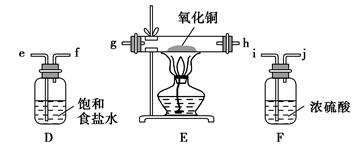
��16�֣�ij��ѧ��ȤС�����ʵ�飬��Ũ������ͭ��Ӧ�Ƶ�SO2���������ʵ��̽����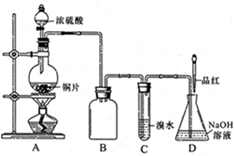
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ
��_______��װ��B��������_______��
���װ��C��Ŀ������֤SO2��
_______________�ԣ�D��NaOHȫ��ת��Ϊ
NaHSO3�ı�־��____________��
��3����Ӧ����ʱ��ƿ��Cu��ʣ�࣬ij
ͬѧ��ΪH2SO4Ҳ��ʣ�࣬�����������ʵ
�鷽�����ⶨʣ��H2SO4����������ȴ����
��ϡ�ͺ��������ʵ�飬�ܴﵽĿ����________������ţ���
a�� ������к͵ζ����ⶨ b�� ������Zn��Ӧ����������H2�����
c�� ��PH�Ʋ���ҺPHֵ d�� ������BaCl2��Һ��Ӧ���������ɳ���������
��4����Dƿ����NaHSO3��Һ�м���Ư����Һ����Ӧ�����ֿ��������
I�� HSO3-��ClO-�պ÷�Ӧ�ꣻ II�� Ư�۲��㣻 III�� Ư�۹���
ͬѧ�Ƿֱ�ȡ���������Һ���Թ��У�ͨ������ʵ��ȷ���÷�Ӧ������һ���������������±���
| ʵ����� | ʵ����� | ���� | ��Ӧ�Ŀ������ |
| �� | �μ��������۵⻯����Һ���� | | III |
| �� | �μ������غ�ɫ��KI3��Һ���� | | II |
| �� | �������������KMNO4��Һ���� | ��Һ����ɫ | |
| �� | ���뼸С��CaCO3���� | �����ݲ��� | |
ij�о���ѧϰС��Ϊ��̽��þ������ˮ��Ӧ�Ļ�����������������ʵ�飺
�ٽ�þ��Ͷ����ˮ�У�δ���κ�����
�ڽ�þ�۷�����ˮ�У��۲쵽ֻ�ǿ�ʼʱ���������������ݣ�����ˮ����ɫ����ȥ��
�۽�þ�۷���Һ���У�δ�۲쵽�κ���������
��������þ�۵�Һ���еμӼ���ˮ���۲쵽��ĺ���ɫ�ܿ���ȥ��
�����й���þ����ˮ�ķ�Ӧ������������ȷ���ǣ� ��
| A��þ��ֱֻ������ˮ�е��巴Ӧ |
| B��þ��ֻ����ˮ�е��ᷴӦ |
| C������������������������þ����ˮ��Ӧ�õ� |
| D��þ����ˮ�Ĵ������巢����Ӧ |

 2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��
2CuCl2��O2�ⶨͭ�Ľ������ԭ���������ɹ�ѡ���װ����ͼ��ʾ��