��Ŀ����
����Ŀ����ҵ�Ͽ������ﴦ����KCN�ķ�ˮ����һ������������������������£���KCNת����KHCO3��NH3�����pH : 6.7��7.2)���ڶ����ǰѰ�ת��Ϊ���NH3+202![]() HNO3+H2O
HNO3+H2O
�����������գ�
��1��д����һ����Ӧ�Ļ�ѧ��Ӧ����ʽ_____________���ڶ�����Ӧ�Ļ�ԭ������_____________ ����д��ѧʽ����
��2����KCN�У����ڶ�������ԭ�Ӱ뾶����Ԫ����_____����ԭ���������ӵ��˶�״̬��_______�֡�ˮ�ĵ���ʽ��________��
��3���Ƚ�̼�͵�Ԫ�طǽ�����ǿ������ѧ��Ӧ����ʽΪ_____________��
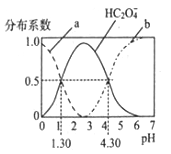
��4�������£�0.lmol/LK2CO3��KCN��KHCO3��Һ���ʼ�����pH���μ�С���ں������ʵ�����KCN��KHCO3�����Һ�У������ӣ���OH-��Ũ���ɴ�С��˳����_____________��
��5����ҵ�ϻ�������������������KCN�ķ�ˮ��KCN+2KOH+Cl2=KOCN+2KCl+H2O��2KOCN+4KOH+3Cl2��N2+6KCl+2CO2+2H2O��������ȣ����ﴦ�������ŵ���ȱ���ǣ���дһ������
�ŵ㣺________��ȱ�㣺__________________��
���𰸡� 2KCN+O2+4H2O![]() 2KHCO3+2NH3 (2�֣�HNO3 H2O ̼(C) 5
2KHCO3+2NH3 (2�֣�HNO3 H2O ̼(C) 5 ![]() NaHCO3+HNO3=NaNO3+CO2��+H2O c(HCO3-)>c(CN-)>c(CO32-) ������Һ��й©�� ������Ӧ�Բ�ȡ�
NaHCO3+HNO3=NaNO3+CO2��+H2O c(HCO3-)>c(CN-)>c(CO32-) ������Һ��й©�� ������Ӧ�Բ�ȡ�
����������1�����ݵ��ӵ�ʧ�غ��ԭ���غ��֪��Ӧ�ﻹ��ˮ�����ε�һ����Ӧ�Ļ�ѧ��Ӧ����ʽΪ2KCN+O2+4H2O![]() 2KHCO3+2NH3���ڶ�����Ӧ������������������ԭ������HNO3��H2O����2����KCN�У����ڶ����ڵ���C��N��ͬ������������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶����Ԫ����C����ԭ���������5�����ӣ����ε��ӵ��˶�״̬��5�֡�ˮ�ǹ��ۻ��������ʽ��
2KHCO3+2NH3���ڶ�����Ӧ������������������ԭ������HNO3��H2O����2����KCN�У����ڶ����ڵ���C��N��ͬ������������ԭ�Ӱ뾶��С����ԭ�Ӱ뾶����Ԫ����C����ԭ���������5�����ӣ����ε��ӵ��˶�״̬��5�֡�ˮ�ǹ��ۻ��������ʽ��![]() ����3����������̼�����Ʒ�Ӧ����̼�ᣬ˵����Ԫ�صķǽ�����ǿ��̼Ԫ�أ���ѧ��Ӧ����ʽΪNaHCO3+HNO3=NaNO3+CO2��+H2O����4��������0.lmol/LK2CO3��KCN��KHCO3��Һ���ʼ�����pH���μ�С��˵��������ӵ�ˮ��̶����μ�С�������ں������ʵ�����KCN��KHCO3�����Һ�У������ӣ���OH-��Ũ���ɴ�С��˳����c(HCO3-)>c(CN-)>c(CO32-)����5�����ݷ�Ӧ�ķ���ʽ���ж����ﴦ�������ŵ��ǣ���ȱ����������Ӧ�Բ�ȡ�
����3����������̼�����Ʒ�Ӧ����̼�ᣬ˵����Ԫ�صķǽ�����ǿ��̼Ԫ�أ���ѧ��Ӧ����ʽΪNaHCO3+HNO3=NaNO3+CO2��+H2O����4��������0.lmol/LK2CO3��KCN��KHCO3��Һ���ʼ�����pH���μ�С��˵��������ӵ�ˮ��̶����μ�С�������ں������ʵ�����KCN��KHCO3�����Һ�У������ӣ���OH-��Ũ���ɴ�С��˳����c(HCO3-)>c(CN-)>c(CO32-)����5�����ݷ�Ӧ�ķ���ʽ���ж����ﴦ�������ŵ��ǣ���ȱ����������Ӧ�Բ�ȡ�

����Ŀ��ʵ��������4��ҩƷ�����Ѿ����������Ʒ��
ҩƷ�� | �׳� | �ҳ� | ���� | ���� |
ҩƷ | ���ᣬ���� | �������ƣ��������� | ���ף��� | ͭ��п |
ʵ�����¹���һЩ�⣬Ӧ�ý���Щ����ڣ� ��
A.�׳�
B.�ҳ�
C.����
D.����